��Ŀ����
����Ŀ����������������ԣ����Ժ��Ҵ�����������Ӧ���ɱ���������������������(�ܶ�1.05gcm-3)����ˮ����ζ������������ˮ�㾫�����쾫�ͣ�Ҳ��������ʳƷ�Լ������л��ϳ��м���ȡ��Ʊ������������Ĺ������£�
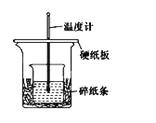
I.�Ʊ��ֲ�Ʒ����ͼ��ʾװ���У���50mlԲ����ƿ�м���8.0g������(Mr=122)��20ml�Ҵ�(Mr=46���ܶ�0.79gcm-3)��15ml�����顢3mlŨ���ᣬҡ�ȣ��ӷ�ʯ���ڷ�ˮ���м�ˮ����ͨ����ˮ��ˮԡ����Լ2h����Ӧ������ɡ���¼��������������������ʹ�(�ӷ�ˮ���зų�)��
�ֲ�Ʒ��������ˮ30ml�������������NaHCO3����Һ��Ȼ��ˮ����20mlʯ���ѷ�������ȡ���ϲ��л��㣬����ˮ����þ�������ʯ���ѣ����Ⱦ����ռ�210~213����֡�
����������£�
�е�(�棬1atm) | ||||||
������ | ���������� | ʯ���� | ˮ | �Ҵ� | ������ | ������ |
249 | 212.6 | 40~80 | 100 | 78.3 | 80.75 | 62.6 |
����������Ϣ��װ��ͼ�ش�����ʵ�����Ʊ��й����⣺
��1��д���Ʊ�������������Ӧ�Ļ�ѧ����ʽ_____________________________________��
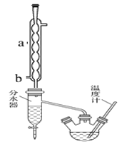
����a������______________������ˮ�Ľ�ˮ��Ϊ______����a��b����
��2���ڱ�ʵ���п�����߱������������ʵķ����У�_________________________________��
A.���뻷�����γ�ˮ![]() �Ҵ�
�Ҵ�![]() ��������Ԫ������������Ӧ���������ɵ�ˮ
��������Ԫ������������Ӧ���������ɵ�ˮ
B.�ӹ������Ҵ�
C.ʹ�÷�ˮ����ʱ��������ɵ�ˮ
��3���������ʵ�������жϷ�Ӧ�ѻ������_____________________________________��
��4������NaHCO3������______________________________���������NaHCO3��ʵ�������Һ���õ�����Ҫ��������Ϊ____________________��
���𰸡�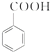 +CH3CH2OH
+CH3CH2OH 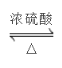
 +
+![]() (����)������ b ABC ���Ȼ�������ˮ����ˮλ�������� �к������δ��Ӧ�ı����� ��Һ©��
(����)������ b ABC ���Ȼ�������ˮ����ˮλ�������� �к������δ��Ӧ�ı����� ��Һ©��
��������
��1����������Ҵ���Ӧ���ɱ�����������ˮ������װ��ͼ�ж����������ƣ�����ˮ����ˮ����Ϊ���½��ϳ�����
��2����������Ҵ���Ӧ���ɱ�����������ˮ�ķ�ӦΪ���淴Ӧ������Ӱ��ƽ���ƶ������ؿ�ȷ����߱������������ʵķ�����
��3�����ݷ�ˮ����ˮλ�жϷ�Ӧ�Ƿ������ɣ�
��4��NaHCO3�ܺ��ᷴӦ���������NaHCO3��ʹ�÷�Һ©�����з�Һ��
��1�����������Ϣ֪����������Ҵ���Ӧ���ɱ�����������ˮ����ѧ��Ӧ����ʽΪ�� +CH3CH2OH
+CH3CH2OH 
 +
+![]() ������װ��ͼ��֪����a������Ϊ�����ܣ�Ϊʹ����Ч����ý�ˮ��Ϊb��
������װ��ͼ��֪����a������Ϊ�����ܣ�Ϊʹ����Ч����ý�ˮ��Ϊb��
��2����������Ҵ���Ӧ���ɱ�����������ˮ�ķ�ӦΪ���淴Ӧ�����Լӹ������Ҵ���ʹ�÷�Һ����ʱ�����ӳ����ɵ�ˮ������������Ҵ���ʹ�÷�Һ����ʱ�����ӳ����ɵ�ˮ������߱������������ʣ���ABC��ȷ��
��3��������Ҵ���Ӧ���ɱ�����������ˮ����ˮ���п��ռ���ˮ�������жϷ�Ӧ�ѻ�����ɵķ����ǣ����Ȼ�������ˮ����ˮλ����������
��4��̼�����ƾ��м��ԣ��ܺ�����ͱ����ᷴӦ�����Σ����Լ���̼�����Ƶ�Ŀ�����к������δ��Ӧ�ı����ᣬ���˹���NaHCO3���Һ������Ҫ�÷�Һ©����

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ������ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����̽����ʵ�飬�ⶨ��Ӧ������SO2��SO3��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
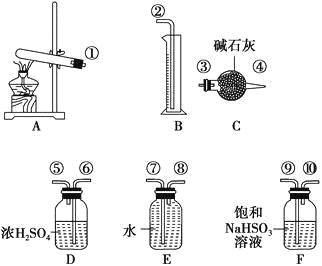
[�������]
��.��������ijɷֿ���ֻ��SO3һ�֣�
��.��������ijɷֿ��ܺ���_______���֣�
��.��������ijɷֿ��ܺ���_______���֡�
[ʵ��̽��]
ʵ����������ԡ���֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
(1)������װ̽��ʵ���װ�ã����������ҵķ��������ӿڵ�����˳��Ϊ�١��������ޡ��ݡ�_______��_______��_______��_______����(��ӿ����)��
(2)��ʵ�����ʱB����Ͳû���ռ���ˮ����֤������_______��ȷ��
(3)������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
ʵ��С�� | ��ȡCuSO4������/g | װ��C���ӵ�����/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
һ | 6.4 | 2.56 | 448 |
�� | 6.4 | 2.56 | 224 |
��ͨ�����㣬�ƶϳ���һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺_______________________________________________________��
�ڶ�С�飺_______________________________________________________��
����Ŀ��ijʵ���ҷ�Һ��![]() ��Na+��Fe3+��Cr3+��
��Na+��Fe3+��Cr3+��![]() ��
��![]() �����ӣ���ͨ���������̱��Ϊ���Ʊ�K2Cr2O7��
�����ӣ���ͨ���������̱��Ϊ���Ʊ�K2Cr2O7��
��֪��
(a)![]() ��
��![]()
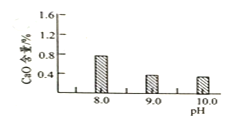
(b)����������������������pH�������
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 2.7 | 3.7 |
Cr3+ | 4.9 | 6.8 |
��ش�
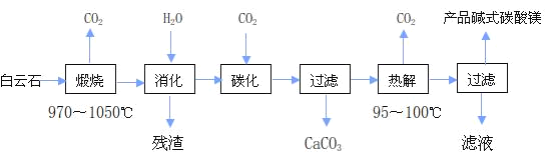
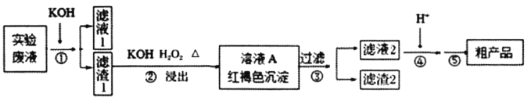
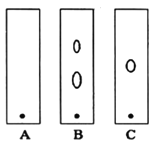
(1)ijͬѧ����ֽ�������жϲ���ټ���KOH�����Ƿ���ʡ��ڼ���һ����KOH��Һ����ëϸ��ȡ��������������ɫ��չ������Ѭ��İߵ���ͼ��ʾ������KOH���ʺϵ�ʵ������(ʵ��˳���Ѵ���)________��C�İߵ���ɫΪ________��
(2)����ں�Cr���ʷ�������Ҫ��Ӧ�����ӷ���ʽΪ________________________��
(3)������װ���У���Ӧѡ��________��(����)
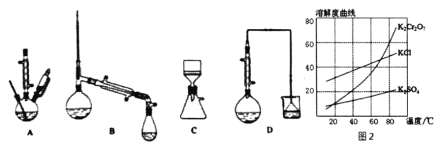
(4)�������ʵ��ܽ��������ͼ2������ݿ����õ����в��ֲ�����a�����������ִ������壬ֹͣ���ȣ�b����ȴ�����£�c����������Һ���־�Ĥ��ֹͣ���ȣ�d��ϴ�ӣ�e�����ȹ��ˣ�f�����ˡ���ѡ����ʲ�������ȷ˳��________��
(5)������к��ʵ�ϴ�Ӽ���________(����ˮ�Ҵ��������Ҵ�-ˮ���Һ��������ˮ��������ˮ��)��
(6)ȡmg�ֲ�Ʒ���250mL��Һ��ȡ25.00mL����ƿ�У���cmol��L-1��(NH4)2Fe(SO4)2����Һ�ζ�(���ʲ���Ӧ)�����ı�(NH4)2Fe(SO4)2��ҺVmL����ôֲ�Ʒ��K2Cr2O7�Ĵ���Ϊ________��