��Ŀ����
(1)�ڷ�ӦA(g)��3B(g)=2C(g)�У���������A��ʾ�ĸ÷�Ӧ�Ļ�ѧ��Ӧ����Ϊ0.2 mol��L��1��min��1����������B��ʾ�˷�Ӧ�Ļ�ѧ��Ӧ����Ϊ________mol��L��1��min��1��
(2)��2 L���ܱ������У�����2 mol N2��3 mol H2����һ�������·�����Ӧ��3 s����N2Ϊ1.9 mol������H2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ________________��
(3)��10 mol A��5 mol B�����ݻ�Ϊ10 L���ܱ������У�ij�¶��·�����Ӧ��3A(g)��B(g)??2C(g)�������2 s�ڣ�����A��ƽ������Ϊ0.06 mol��L��1��s��1������2 sʱ����������________mol A����ʱC�����ʵ���Ũ��Ϊ______��
(2)��2 L���ܱ������У�����2 mol N2��3 mol H2����һ�������·�����Ӧ��3 s����N2Ϊ1.9 mol������H2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ________________��
(3)��10 mol A��5 mol B�����ݻ�Ϊ10 L���ܱ������У�ij�¶��·�����Ӧ��3A(g)��B(g)??2C(g)�������2 s�ڣ�����A��ƽ������Ϊ0.06 mol��L��1��s��1������2 sʱ����������________mol A����ʱC�����ʵ���Ũ��Ϊ______��
(1)0.6��(2)0.05 mol��L��1��s��1 (3)8.8��0.08 mol��L��1
(1)���ݻ�ѧ����ʽ�еĻ�ѧ��������ϵ��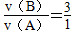 ��v(B)��3v(A)��3��0.2 mol��L��1��min��1��0.6 mol��L��1��min��1��
��v(B)��3v(A)��3��0.2 mol��L��1��min��1��0.6 mol��L��1��min��1��
(2)�ⷨһ��3 s�����ĵ�N2�����ʵ���Ϊ2 mol��1.9 mol��0.1 mol�����ݻ�ѧ����ʽN2��3H2 2NH3�����Լ����3 s�����ĵ�H2�����ʵ���Ϊ0.3 mol�����ݻ�ѧ��Ӧ���ʵļ��㹫ʽ��v(H2)��
2NH3�����Լ����3 s�����ĵ�H2�����ʵ���Ϊ0.3 mol�����ݻ�ѧ��Ӧ���ʵļ��㹫ʽ��v(H2)��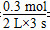 ��0.05 mol��L��1��s��1��
��0.05 mol��L��1��s��1��
�ⷨ�����ȼ������N2��ʾ�Ļ�ѧ��Ӧ����v(N2)�� mol��L��1��s��1���ٸ��ݻ�ѧ����ʽ�Ļ�ѧ��������ϵ���������H2��ʾ�Ļ�ѧ��Ӧ����v(H2)��3v(N2)��0.05 mol��L��1��s��1��
mol��L��1��s��1���ٸ��ݻ�ѧ����ʽ�Ļ�ѧ��������ϵ���������H2��ʾ�Ļ�ѧ��Ӧ����v(H2)��3v(N2)��0.05 mol��L��1��s��1��
(3)���������������� 3A(g)������B(g) ��2C(g)
��2C(g)
��ʼŨ��/(mol��L��1) 1.0 0.5 0
ת��Ũ��/(mol��L��1)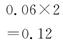 0.04 0.08
0.04 0.08
2 sʱŨ��/(mol��L��1)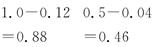 0.08
0.08
��2 sʱ��n(A)��0.88 mol��L��1��10 L��8.8 mol
c(C)��0.08 mol��L��1��
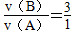 ��v(B)��3v(A)��3��0.2 mol��L��1��min��1��0.6 mol��L��1��min��1��
��v(B)��3v(A)��3��0.2 mol��L��1��min��1��0.6 mol��L��1��min��1��(2)�ⷨһ��3 s�����ĵ�N2�����ʵ���Ϊ2 mol��1.9 mol��0.1 mol�����ݻ�ѧ����ʽN2��3H2
 2NH3�����Լ����3 s�����ĵ�H2�����ʵ���Ϊ0.3 mol�����ݻ�ѧ��Ӧ���ʵļ��㹫ʽ��v(H2)��
2NH3�����Լ����3 s�����ĵ�H2�����ʵ���Ϊ0.3 mol�����ݻ�ѧ��Ӧ���ʵļ��㹫ʽ��v(H2)��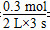 ��0.05 mol��L��1��s��1��
��0.05 mol��L��1��s��1���ⷨ�����ȼ������N2��ʾ�Ļ�ѧ��Ӧ����v(N2)��
 mol��L��1��s��1���ٸ��ݻ�ѧ����ʽ�Ļ�ѧ��������ϵ���������H2��ʾ�Ļ�ѧ��Ӧ����v(H2)��3v(N2)��0.05 mol��L��1��s��1��
mol��L��1��s��1���ٸ��ݻ�ѧ����ʽ�Ļ�ѧ��������ϵ���������H2��ʾ�Ļ�ѧ��Ӧ����v(H2)��3v(N2)��0.05 mol��L��1��s��1��(3)���������������� 3A(g)������B(g)
 ��2C(g)
��2C(g)��ʼŨ��/(mol��L��1) 1.0 0.5 0
ת��Ũ��/(mol��L��1)
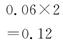 0.04 0.08
0.04 0.082 sʱŨ��/(mol��L��1)
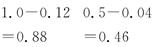 0.08
0.08��2 sʱ��n(A)��0.88 mol��L��1��10 L��8.8 mol
c(C)��0.08 mol��L��1��

��ϰ��ϵ�д�
 ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
�����Ŀ
 2NO2(g)��ϵ�У�n(NO)��ʱ��仯���±���
2NO2(g)��ϵ�У�n(NO)��ʱ��仯���±���
 2B(g)����H��-a kJ��mol��1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
2B(g)����H��-a kJ��mol��1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
 yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
 2 CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
2 CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��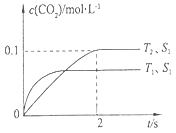

 3C��4D��Ӧ�У���ʾ�÷�Ӧ����������(����)��
3C��4D��Ӧ�У���ʾ�÷�Ӧ����������(����)�� 2HI��g�������¶�һ������H2��g����I2��g����ʼ��Ӧ������˵����ȷ���ǣ� ��
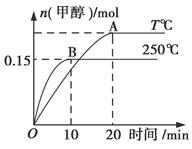
2HI��g�������¶�һ������H2��g����I2��g����ʼ��Ӧ������˵����ȷ���ǣ� �� CH3OH(g)����H��a kJ/mol����ͼ��ʾ�����з�������ȷ����(����)
CH3OH(g)����H��a kJ/mol����ͼ��ʾ�����з�������ȷ����(����)