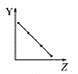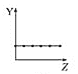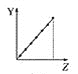��Ŀ����
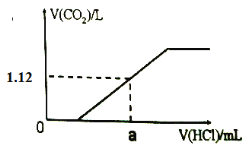
����Ŀ��25��ʱ��Ũ�Ⱦ�Ϊ0.100 mol/L��HA��Һ��BOH��Һ��20.00 mL���ֱ���0.100 mol/L NaOH��Һ��0.100 mol/L ������еζ����ζ�������pH��μ���Һ����仯��ϵ��ͼ����ͼ�����pH=7�����¶Գơ�����˵���������
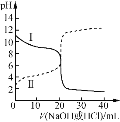
A.���ߢ��ʾ����μӵ�BOH��Һ�Ĺ��̣����ü�����ָʾ��
B.Ka(HA) =Kb(BOH)�������Ӧ������V< 20.00 mL
C.���ߢμ���Һ��10.00 mLʱ��c(BOH) > c(Cl��) > c(OH��) >c(H+)
D.���ߢμ���Һ��5.00~10.00 mLʱ��c(A��) +c(OH��) =c(H+) +c(Na+)
���𰸡�C
��������
����ͼʾ�����ߢ�ʼ�ʼ��ԣ�Ϊ����μӵ�BOH��Һ�Ĺ��̣����ߢ�ʼ�����ԣ�ΪNaOH��Һ�μӵ�HA��Һ�Ĺ��̡�
A. ���ȱ�ɫ��Χ3.1~4.4������ζ�BOHʱ����ѡ�ü��ȣ��յ��ɻ�ɫ��Ϊ��ɫ�����Կ��ü�����ָʾ����A��ȷ��
B. ��ͼ�����pH=7�����¶Գƣ�������Һ��ˮ��������������Ӻ����������Ӷ���ͬ����Һ�е�����Ũ����ͬ����Ka(HA) =Kb(BOH)������ʱpH=7����Ϊ����ζ�BOHʱ��BClΪǿ�������Σ���V= 20.00 mL����Һ�����ԣ�pH<7����pH=7���������������< 20.00 mL��ͬ����ΪNaOH�ζ�HAʱ�����������������< 20.00 mL�����Զ�Ӧ������V< 20.00 mL��B��ȷ��
C. ���ߢ�Ϊ����ζ�BOH���μ���Һ��10.00 mLʱ����Һ������Ϊ�����ʵ�����BOH��BCl������BOHΪ�������Ũ���У�c(Cl��) >c(BOH) > c(OH��) >c(H+)��C����
D. ���ߢ�ΪNaOH�ζ�HA���μ���Һ��5.00~10.00 mLʱ����Һ������ΪHA��NaA�����ݵ���غ��ϵ����c(A��) +c(OH��) =c(H+) +c(Na+)��D��ȷ��
��ѡC��

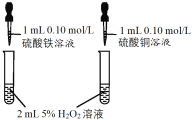
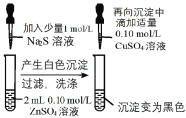
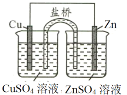
����Ŀ��CuSO4��Һ��ʵ�����г����Լ���������CuSO4��Һ�й�ʵ��IJ����ͽ��۶�һ����ȷ����
ѡ�� | A | B | C | D |
ʵ����� |
ǰ�߲������ݸ��� |
|
CuƬ�Ϸ�����ԭ��Ӧ |
����CuSO4��Һ |
���� | Fe3+��Ч������Cu2+ | Ksp(CuS) < Ksp(ZnS) | CuƬΪ������Ӧ�� | �ɻ�õ������� |
A.AB.BC.CD.D