��Ŀ����
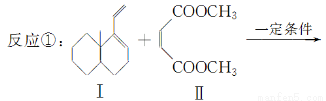
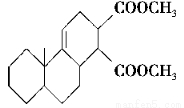
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ������ᷴӦ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
��.����ʯ�к������IJⶨ������ʵ����̲��������벹��������
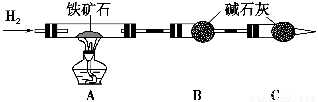
��1������ͼ��װ��������______________________________________________��
��2����8.0 g����ʯ����Ӳ�ʲ���������װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
��3������˵����ܿڴ����ϵػ���ͨ��H2��____________________________��
��ȼA���ƾ��ƣ�
��4����ַ�Ӧ���������ƾ�����________________________________________��
��5����÷�Ӧ��װ��B����2.25 g��������ʯ�����İٷֺ���Ϊ________��
��.����ʯ�к������IJⶨ���������¡�
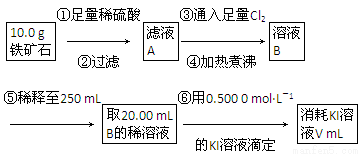
��1������������������___________________________________________��
��2�����������õ��IJ����������ձ�����ͷ�ιܡ�250 mL����ƿ��________��
��3�������йز������IJ�����˵����ȷ����________��
a����Ϊ��ˮΪ��ɫ�����Եζ������������ָʾ��
b����ƿ����Ҫ�ô���Һ��ϴ
c���ζ������п����õ�����Һ��ָʾ��
d���ζ����������۾�ע�ӵζ�����Һ��仯
e���ζ���������30 s����Һ���ָ�ԭ������ɫ���ٶ���
f���ζ����������ζ��ܼ��첿������������ⶨ���ƫ��
��4�����ζ�����������0.500 0 mol��L��1 KI��Һ20.00 mL��������ʯ�����İٷֺ���Ϊ________��
��.�����������������������ʯ������������Ļ�ѧʽΪ________��
��.��1�����װ�õ������ԡ���3����װ��C���ڴ������鴿��4���ٳ���ͨ��������Ӳ�ʲ�������ȫ��ȴ����5��25.0%
��.��1��������Һ���ܽ�Ĺ���Cl2����2������������3��be����4��70.0%
��.Fe4O5
�����������⿼������ʯ������������Ļ�ѧʽ��̽����
��.ʵ�鿪ʼǰһ��Ҫ�ȼ��װ�õ�����������װ��ҩƷ���ڵ�ȼA���ƾ���֮ǰһ��Ҫ�ȼ��������Ĵ��ȣ������ƾ��ƺ���һ��Ҫ��ͨ������һ��ʱ����ֱ��Ӳ�ʲ�������ȴ���������Է������±�������װ��B���ӵ�������Ϊ����ˮ������������m��O����2.25 g��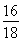 ��2.0 g����Ԫ�ص���������Ϊ
��2.0 g����Ԫ�ص���������Ϊ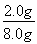 ��100%��25.0%����.��1����Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ����������2������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢��������3����ˮ�Ļ�ɫ��������a���ζ�ʱ������Ӧ��2Fe3����2I��=2Fe2����I2���ζ�ʱ����������������Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ����������ζ��ܼ��첿����������������KI��Һ������ƫ�����ⶨ���ƫ����f����
��100%��25.0%����.��1����Ϊ����KI��Һ���еζ���������Ҳ����KI��Ӧ��Ӱ��ʵ���������Ա����ȥ��Һ���ܽ����������2������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�ò��������衢��������3����ˮ�Ļ�ɫ��������a���ζ�ʱ������Ӧ��2Fe3����2I��=2Fe2����I2���ζ�ʱ����������������Һ���������ζ��յ�ʱû����ɫ�仯�����Բ����õ�����ָʾ����c���ζ�������Ҫ�۲���ƿ����Һ��ɫ�ı仯������ע�ӵζ�����Һ��仯��d���ζ����������ζ��ܼ��첿����������������KI��Һ������ƫ�����ⶨ���ƫ����f����
��4������2Fe3����2I��=2Fe2����I2��֪10.0 g����ʯ����n��Fe����0.500 0 mol��L��1��0.02 L��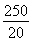 ��0.125 mol��m��Fe����7.0 g������Ԫ�ص���������Ϊ70.0%����.��������������n��Fe����n��O����
��0.125 mol��m��Fe����7.0 g������Ԫ�ص���������Ϊ70.0%����.��������������n��Fe����n��O����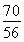 ��
�� ��4��5�������仯ѧʽΪFe4O5��
��4��5�������仯ѧʽΪFe4O5��

����ͭ���ȷֽ���������ͭ�������������¶Ȳ�ͬ������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����̽����ʵ�����ⶨ��Ӧ������SO2��SO3��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
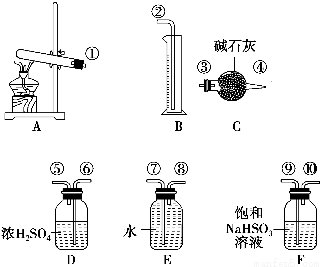
[�������]
��.��������ijɷֿ���ֻ��SO3һ�֣�
��.��������ijɷֿ��ܺ���________���֣�
��.��������ijɷֿ��ܺ���________���֡�
[ʵ��̽��]
ʵ����������ԡ���֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1��������װ̽��ʵ���װ�������������ҵķ������������ӿڵ�����˳��Ϊ��������������������________��________��________��________��������ӿ���ţ���
��2����ʵ�����ʱB����Ͳû���ռ���ˮ����֤������________��ȷ��
��3��������ʵ��С����и�ʵ�������ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
ʵ�� С�� | ��ȡCuSO4 ������/g | װ��C���� ������/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
һ | 6.4 | 2.56 | 448 |
�� | 6.4 | 2.56 | 224 |
��ͨ���������ƶϳ���һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺________________________________________________________��
�ڶ�С�飺________________________________________________________��