��Ŀ����
��12�֣��ڻ���ƽ�����װ��ǿ��ԭ���£�N2H4����ǿ��������H2O2���������ǻ��ʱ��������������N2��ˮ���������ų������ȡ���֪0.4molҺ̬�º�����H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65kJ��������
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ__________________________________________��
��2����֪H 2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ��
2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ��
��3��������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ����ͻ�����ŵ���________________________��
��4����֪N2(g)+2O2(g)="===2" NO2(g)����H="+67.7" kJ��mol-1�� N2H4(g)+O2(g)="==" N2(g)+2H2O (g)����H=��534 kJ��mol-1�����ݸ�˹����д������NO2��ȫ��Ӧ���ɵ�������̬ˮ���Ȼ�ѧ����ʽ_____________________��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ__________________________________________��
��2����֪H
 2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ��
2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ����3��������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ����ͻ�����ŵ���________________________��
��4����֪N2(g)+2O2(g)="===2" NO2(g)����H="+67.7" kJ��mol-1�� N2H4(g)+O2(g)="==" N2(g)+2H2O (g)����H=��534 kJ��mol-1�����ݸ�˹����д������NO2��ȫ��Ӧ���ɵ�������̬ˮ���Ȼ�ѧ����ʽ_____________________��
��

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
 2SO3��g������H =" a" kJ/mol����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99kJ����ش�
2SO3��g������H =" a" kJ/mol����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g����ȫת��Ϊ1mol SO3��g������99kJ����ش�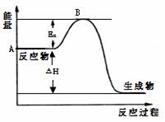
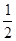 O2(g)=H2O(l) ��H3="-285.8KJ/mol "
O2(g)=H2O(l) ��H3="-285.8KJ/mol "  CH3OH (g) ��H 1����Ӧ��
CH3OH (g) ��H 1����Ӧ��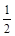 O2(g)��CO2(g) ��H 2����283 kJ��mol��
O2(g)��CO2(g) ��H 2����283 kJ��mol�� 1 (��Ӧ��)
1 (��Ӧ��)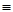 O
O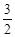 O2(g)��CO2(g)��2H2O(g) ��H4
O2(g)��CO2(g)��2H2O(g) ��H4
 ѧʽ��
ѧʽ�� �Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��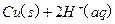 ====
====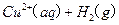
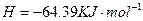
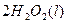 ====
====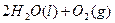
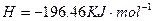
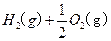 ="==="
="===" 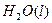
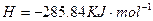
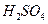 ��Һ��
��Һ��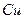 ��
��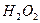 ��Ӧ����
��Ӧ����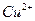 ��
��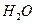 ���Ȼ�����ʽΪ ��
���Ȼ�����ʽΪ ��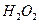 ��3.0
��3.0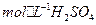 �Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
�Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����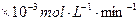 ��[:.....]
��[:.....]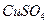 ��Һ�м���һ������
��Һ�м���һ������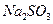 ��
��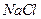 ��Һ�����ȣ�����
��Һ�����ȣ�����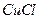 �������Ʊ�
�������Ʊ� �ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;����
�ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;���� Ũ�ȵ���__________ (ѡ����ĸ)��
Ũ�ȵ���__________ (ѡ����ĸ)��


 �����£�
�����£�
 ,��ƽ�ⳣ��K=13.3��
,��ƽ�ⳣ��K=13.3�� ����
���� =_______________(������λ��Ч����)��
=_______________(������λ��Ч����)��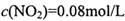 ��
�� ����ı��������____________________
����ı��������____________________