Ő‚ńŅńŕ»›
£®10∑÷£©∑Ōĺ…”°ňĘĶÁ¬∑įŚĶńĽō ’ņŻ”√Ņ… ĶŌ÷◊ ‘Ŕ…ķ£¨≤Ęľű…ŔőŘ»ĺ°£∑Ōĺ…”°ňĘĶÁ¬∑įŚĺ≠∑Řňť∑÷ņŽ£¨ń‹Ķ√ĶĹ∑«Ĺū Ű∑Řń©ļÕĹū Ű∑Řń©°£
£®1£©£®2∑÷£©Ō¬Ń–ī¶ņŪ”°ňĘĶÁ¬∑įŚ∑«Ĺū Ű∑Řń©Ķń∑Ĺ∑®÷–£¨≤Ľ∑ŻļŌĽ∑ĺ≥Ī£Ľ§ņŪńÓĶń « £®ŐÓ◊÷ńł£©°£
£®2£©£®2∑÷£©”√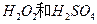 ĶńĽžļŌ»‹“ļŅ…»‹≥Ų”°ňĘĶÁ¬∑įŚĹū Ű∑Řń©÷–ĶńÕ≠°£“—÷™£ļ
ĶńĽžļŌ»‹“ļŅ…»‹≥Ų”°ňĘĶÁ¬∑įŚĹū Ű∑Řń©÷–ĶńÕ≠°£“—÷™£ļ
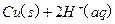 ====
====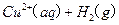
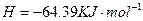
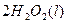 ====
====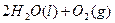
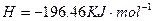
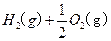 ="==="
="===" 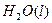
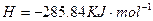
‘ŕ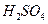 »‹“ļ÷–
»‹“ļ÷–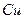 ”Ž
”Ž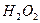 ∑ī”¶…ķ≥…
∑ī”¶…ķ≥…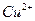 ļÕ
ļÕ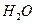 Ķń»»ĽĮ∑Ĺ≥Ő Ĺő™ °£
Ķń»»ĽĮ∑Ĺ≥Ő Ĺő™ °£
£®3£©£®3∑÷£©Ņō÷∆∆šňŻŐűľĢŌŗÕ¨£¨”°ňĘĶÁ¬∑įŚĶńĹū Ű∑Řń©”√10®G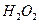 ļÕ3.0
ļÕ3.0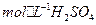 ĶńĽžļŌ»‹“ļī¶ņŪ£¨≤‚Ķ√≤ĽÕ¨ő¬∂»Ō¬Õ≠Ķń∆Ĺĺý»‹Ĺ‚ňŔ¬ £®ľŻŌ¬ĪŪ£©°£
ĶńĽžļŌ»‹“ļī¶ņŪ£¨≤‚Ķ√≤ĽÕ¨ő¬∂»Ō¬Õ≠Ķń∆Ĺĺý»‹Ĺ‚ňŔ¬ £®ľŻŌ¬ĪŪ£©°£
ĶĪő¬∂»łŖ”ŕ40°ś Ī£¨Õ≠Ķń∆Ĺĺý»‹Ĺ‚ňŔ¬ ňś◊Ň∑ī”¶ő¬∂»…żłŖ∂ÝŌ¬ĹĶ£¨∆š÷ų“™‘≠“Ú « °£
£®4£©£®3∑÷£©‘ŕŐŠīŅļůĶń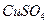 »‹“ļ÷–ľ”»Ž“Ľ∂®ŃŅĶń
»‹“ļ÷–ľ”»Ž“Ľ∂®ŃŅĶń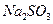 ļÕ
ļÕ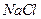 »‹“ļ£¨ľ”»»£¨…ķ≥…
»‹“ļ£¨ľ”»»£¨…ķ≥…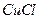 ≥ŃĶŪ°£÷∆Īł
≥ŃĶŪ°£÷∆Īł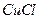 ĶńņŽ◊”∑Ĺ≥Ő Ĺ « °£
ĶńņŽ◊”∑Ĺ≥Ő Ĺ « °£
£®1£©£®2∑÷£©Ō¬Ń–ī¶ņŪ”°ňĘĶÁ¬∑įŚ∑«Ĺū Ű∑Řń©Ķń∑Ĺ∑®÷–£¨≤Ľ∑ŻļŌĽ∑ĺ≥Ī£Ľ§ņŪńÓĶń « £®ŐÓ◊÷ńł£©°£
| A£ģ»»Ń—Ĺ‚–ő≥…»ľ”Õ | B£ģ¬∂Őž∑Ŕ…’ |
| C£ģ◊ųő™”–ĽķłīļŌĹ®÷Ģ≤ńŃŌĶń‘≠ŃŌ | D£ģ÷ĪĹ”ŐӬ٠|
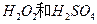 ĶńĽžļŌ»‹“ļŅ…»‹≥Ų”°ňĘĶÁ¬∑įŚĹū Ű∑Řń©÷–ĶńÕ≠°£“—÷™£ļ
ĶńĽžļŌ»‹“ļŅ…»‹≥Ų”°ňĘĶÁ¬∑įŚĹū Ű∑Řń©÷–ĶńÕ≠°£“—÷™£ļ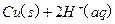 ====
====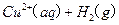
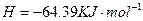
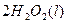 ====
====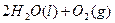
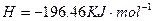
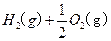 ="==="
="===" 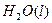
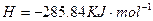
‘ŕ
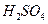 »‹“ļ÷–
»‹“ļ÷–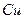 ”Ž
”Ž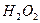 ∑ī”¶…ķ≥…
∑ī”¶…ķ≥…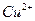 ļÕ
ļÕ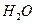 Ķń»»ĽĮ∑Ĺ≥Ő Ĺő™ °£
Ķń»»ĽĮ∑Ĺ≥Ő Ĺő™ °££®3£©£®3∑÷£©Ņō÷∆∆šňŻŐűľĢŌŗÕ¨£¨”°ňĘĶÁ¬∑įŚĶńĹū Ű∑Řń©”√10®G
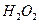 ļÕ3.0
ļÕ3.0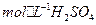 ĶńĽžļŌ»‹“ļī¶ņŪ£¨≤‚Ķ√≤ĽÕ¨ő¬∂»Ō¬Õ≠Ķń∆Ĺĺý»‹Ĺ‚ňŔ¬ £®ľŻŌ¬ĪŪ£©°£
ĶńĽžļŌ»‹“ļī¶ņŪ£¨≤‚Ķ√≤ĽÕ¨ő¬∂»Ō¬Õ≠Ķń∆Ĺĺý»‹Ĺ‚ňŔ¬ £®ľŻŌ¬ĪŪ£©°£| ő¬∂»£®°ś£© | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Õ≠∆Ĺĺý»‹Ĺ‚ňŔ¬ £® 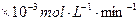 £©[:.....] £©[:.....] | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
£®4£©£®3∑÷£©‘ŕŐŠīŅļůĶń
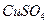 »‹“ļ÷–ľ”»Ž“Ľ∂®ŃŅĶń
»‹“ļ÷–ľ”»Ž“Ľ∂®ŃŅĶń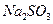 ļÕ
ļÕ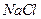 »‹“ļ£¨ľ”»»£¨…ķ≥…
»‹“ļ£¨ľ”»»£¨…ķ≥…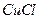 ≥ŃĶŪ°£÷∆Īł
≥ŃĶŪ°£÷∆Īł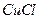 ĶńņŽ◊”∑Ĺ≥Ő Ĺ « °£
ĶńņŽ◊”∑Ĺ≥Ő Ĺ « °££®1£©BD
£®2£©Cu£®S£©+H2O2£®l£©+2H+£®aq£©=====Cu2+(aq)+2H20£®l£©°ųH=-319.68J°§mol-1
£®3£©H2O2∑÷Ĺ‚ňŔ¬ ľ”Ņž
£®4£©2Cu2++SO32-+2Cl-+H2O 2CuCl °ż+SO42-+2H+
2CuCl °ż+SO42-+2H+
£®2£©Cu£®S£©+H2O2£®l£©+2H+£®aq£©=====Cu2+(aq)+2H20£®l£©°ųH=-319.68J°§mol-1
£®3£©H2O2∑÷Ĺ‚ňŔ¬ ľ”Ņž
£®4£©2Cu2++SO32-+2Cl-+H2O
 2CuCl °ż+SO42-+2H+
2CuCl °ż+SO42-+2H+¬‘

Ń∑Ōį≤ŠŌĶŃ–īūįł
 √Ż–£ŅőŐ√ŌĶŃ–īūįł
√Ż–£ŅőŐ√ŌĶŃ–īūįł
ŌŗĻōŐ‚ńŅ
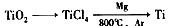
 2O(l)====H2O(g)£Ľ°ųH=+44kJ°§mol-1£¨‘Ú16 g“ļŐ¨Ž¬»ľ…’…ķ≥…Ķ™∆ÝļÕ“ļŐ¨ňģ Ī£¨∑Ň≥ŲĶń»»ŃŅ «________kJ°£
2O(l)====H2O(g)£Ľ°ųH=+44kJ°§mol-1£¨‘Ú16 g“ļŐ¨Ž¬»ľ…’…ķ≥…Ķ™∆ÝļÕ“ļŐ¨ňģ Ī£¨∑Ň≥ŲĶń»»ŃŅ «________kJ°£ O2 ( g )+2H2O ( l)°ųH£Ĺ“Ľ867 kJ°§mol£≠1
O2 ( g )+2H2O ( l)°ųH£Ĺ“Ľ867 kJ°§mol£≠1 £≠1
£≠1