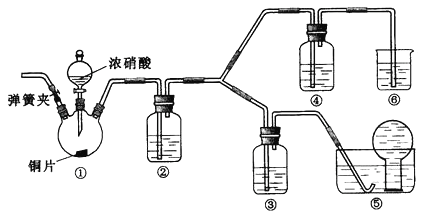
��Ŀ����
����Ŀ��ʵ��������ϩʱ�������¶ȹ��߶���������Ӧ�������Ҵ���Ũ���ᷴӦ���ɶ�����������̼��ˮ������̿�ڣ��������б��Ϊ�����ܵ�ʵ��װ�����һ��ʵ�飬����֤������Ӧ��Ļ�����к��ж�����̼�����������ˮ������


(1)��װ�õı�ű�ʾ������˳��(�������������ҵ�����)__________________��
(2)װ�â��еĹ���ҩƷΪ__________________������֤�IJ�����__________________��������__________________��
(3)װ�â��е�������__________________����֤__________________��װ�â�����ˮ��������__________________��װ�â���Ʒ����Һ����ɫ˵��__________________����ʱʢ��__________________��װ�â��г���__________________��������֤__________________����Ĵ��ڡ�
���𰸡� �ޢݢ٢ڢۢ� ��ˮCuSO4 H2O ��ɫ��ĩ����ɫ Ʒ����Һ��ɫ ��������к���SO2 ����SO2 ���������Ѿ����� ����ʯ��ˮ �������� ������̼
��������������������⿼����ϩ�е�CO2��SO2��ˮ�����ļ��顣CO2Ӧ�ó���ʯ��ˮ���顢SO2��Ʒ����Һ������ˮ��������ˮ����ͭ���顣���ڳ���ʯ��ˮ��Ʒ����Һ�����ˮ��������ˮ�����ļ��飬�����ȼ���ˮ������SO2Ҳ��ʹ�����ʯ��ˮ����ǣ�����CO2�ļ��飬�����ȼ���SO2����SO2�������ټ���CO2�������˳��Ϊ������ˮ����������SO2������SO2������CO2��
��1��װ�ñ�ŵ�����˳��Ϊ���ޢݢ٢ڢۢܡ�
��2��װ�â��еĹ���ҩƷΪ��ˮCuSO4��������֤�IJ�����ˮ�����������ǰ�ɫ��ĩ�����ɫ��
��3��װ�â���Ʒ�����ڼ���SO2��װ�����е�������Ʒ����Һ��ɫ����֤��������к���SO2��װ�â�����ˮ������������SO2����ϩ����Ӧ��Ӧ�Ļ�ѧ����ʽΪ��SO2+Br2+2H2O=H2SO4+2HBr��CH2=CH2+Br2��BrCH2CH2Br��װ�â���Ʒ����Һ��������������SO2�Ƿ������װ�â���Ʒ����Һ����ɫ˵��SO2�Ѿ�������ʢ�г���ʯ��ˮ��װ�â��г��ֻ�����������֤CO2����Ĵ��ڡ�

����Ŀ��һ���¶��£��ڼס��ҡ��������ĸ������ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж�����ȷ����
�� | �� | �� | �� | ||
���������/L | 2 | 2 | 2 | 1 | |
��ʼ���ʵ��� | n(SO2)/mol | 0.40 | 0.80 | 0.80 | 0.40 |
n(O2)/mol | 0.24 | 0.24 | 0.48 | 0.24 | |
SO2��ƽ��ת����% | 80 | ��1 | ��2 | ��3 | |
A. ���з�Ӧ��ƽ�ⳣ��С���� B. ���¶��£��÷�Ӧ��ƽ�ⳣ��KΪ400
C. SO2��ƽ��ת���ʣ���1����2����3 D. ������SO3�����ʵ���Ũ�ȣ�����������