��Ŀ����
����Ŀ��A��B��C��D��E��F�Ǻ˵����������������ֶ���������Ԫ�أ�AԪ�ص�ԭ�Ӻ���ֻ��1�����ӣ�BԪ�ص�ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ�����4��C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��C��Eͬ���塣
(1)B�����ڱ��е�λ��______________________________________________
(2)FԪ�ص�����������Ӧ��ˮ����Ļ�ѧʽΪ___________________________________��
(3)Ԫ��C��D��E�γɵļ����Ӱ뾶��С�����˳��________________________(�����ӷ��ű�ʾ)��
(4)�õ���ʽ��ʾ������D2C���γɹ��̣�__________________________________________________��
C��D�����γɻ�����D2C2��D2C2�к��еĻ�ѧ����_________________________________________��
(5)C��E���⻯��е��ɸߵ���˳���ǣ�_______________________________��
(6)д��̼������E������������Ӧˮ����Ũ��Һ��Ӧ�Ļ�ѧ����ʽ�����õ����ű������ӵ�ת�Ʒ���_______________����ת�Ƶ���Ϊ0.2molʱ����״���·�Ӧ��������_______________L��
(7)��֪E���ʺ�F���ʵ�ˮ��Һ��Ӧ����������ǿ�ᣬ�����ӷ���ʽΪ_________________��
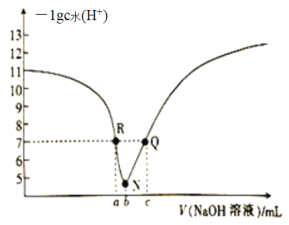
���𰸡��ڶ����ڵ���A�� HClO4 r(Na+) ��r(O2��)�� r(S2��)��Na+ ��O2����S2�� ![]() ���Ӽ������ۼ�(��Ǽ��Լ�) H2O��H2S
���Ӽ������ۼ�(��Ǽ��Լ�) H2O��H2S 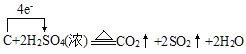 3.36 S��3Cl2��4H2O = 8H+��6Cl����SO42-
3.36 S��3Cl2��4H2O = 8H+��6Cl����SO42-
��������
AԪ�ص�ԭ�Ӻ���ֻ��һ�����ӣ��ɴ˿�֪AΪH������ B������������Ӧˮ����Ļ�ѧʽΪHBO3 ����֪B�����Ϊ+5�ۣ���VA���ٸ���BԪ�ص�ԭ�Ӱ뾶����������������С�ģ��ó�BΪN������CԪ��ԭ�ӵ������������ȴ�����������4���ó�CΪO��C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C����֪D��������Ϊ+1�������ӣ�����DΪNa��C��Eͬ���壬��EΪ������Ԫ�أ��ó�EΪS��F�Ƕ�����Ԫ������SԪ�غ��������Ԫ�أ�Fֻ��ΪCl�����Ͽ�֪AΪH��BΪN��CΪO��DΪNa��EΪS��FΪCl���ݴ˻ش�
��1��BΪ��Ԫ�أ�Nλ�����ڱ��ڶ����ڵ�VA�壬�ʴ�Ϊ���ڶ����ڣ�VA��
��2��FΪClԪ�أ�ClԪ������������ˮ������HClO4���ʴ�Ϊ��HClO4��
��3��Ԫ��C��D��E�γɵļ����ӷֱ�ΪO2����Na+��S2�������Ӳ���������Ӱ뾶�ϴ�������Ų���ͬ�����������Ӱ뾶��ԭ���������������С�����뾶��С����Ϊr(Na+) ��r(O2��)�� r(S2��)���ʴ�Ϊ��r(Na+) ��r(O2��)�� r(S2��)��
��4��D2C��Na2O���õ���ʽ��ʾ�γɹ���Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��D2C2ΪNa2O2�����к��еĻ�ѧ��Ϊ���Ӽ������ۼ����ʴ�Ϊ�����Ӽ������ۼ���
��D2C2ΪNa2O2�����к��еĻ�ѧ��Ϊ���Ӽ������ۼ����ʴ�Ϊ�����Ӽ������ۼ���
��5��C��E���⻯��ֱ�ΪH2O��H2S������H2O����֮�������������·е�Ҫ���ߣ����Էе�H2O��H2S���ʴ�Ϊ��H2O��H2S��
��6��C��Ũ���ᷴӦ����ʽΪ��C+2H2SO4(Ũ) ![]() 2SO2��+CO2��+2H2O�������������������ת��
2SO2��+CO2��+2H2O�������������������ת��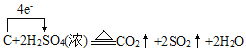 ����ת��4mole-ʱ��������SO2 2mol��CO2 1mol����3mol����״�������Ϊ67.2L����ת��0.2mol����ʱ���������Ϊ
����ת��4mole-ʱ��������SO2 2mol��CO2 1mol����3mol����״�������Ϊ67.2L����ת��0.2mol����ʱ���������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��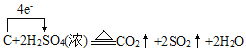 ��3.36L��
��3.36L��
��7��S����ˮ��Ӧ�Ļ�ѧ����ʽΪ3Cl2+S+ 4H2O=6HCl+H2SO4���������ӷ���ʽΪS��3Cl2��4H2O = 8H+��6Cl����SO42-���ʴ�Ϊ��S��/span>3Cl2��4H2O = 8H+��6Cl����SO42-��