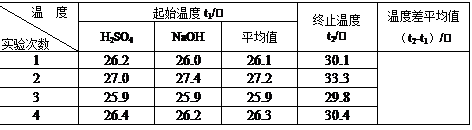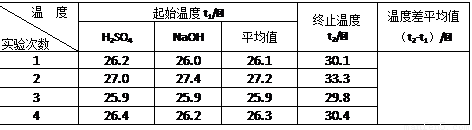��Ŀ����
 ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��������0.50mol/L NaOH��Һ��1����ʵ���д�ԼҪʹ��245mL NaOH��Һ��������Ҫ����NaOH����
��2������NaOH��������Ҫ�����������е�
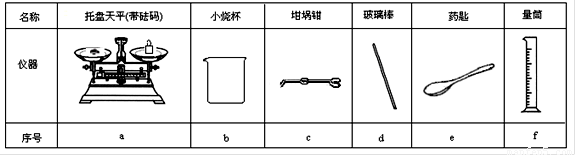
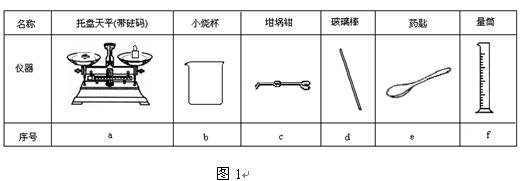
��������ƽ����С�ձ��������ǯ���ܲ�������ҩ�ס�����Ͳ
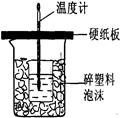
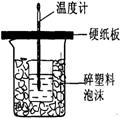
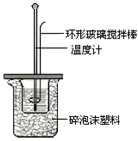
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1������ĭ���ϵ�������
��2��д���÷�Ӧ���Ȼ�ѧ����ʽΪ���к���Ϊ57.3kJ/mol����
��3��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶�ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.3 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.0 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��
a��װ�ñ��¡�����Ч���
b����ȡNaOH��Һ�����ʱ���Ӷ�����
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ�
��2���������ƾ�����ʪ�ԣ�Ӧ����С�ձ��г��������ݳ������������������õ��������ش�
��1�������к��ȵ�ʵ��Ĺؼ��DZ��£�
��2����������кͷ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������д�Ȼ�ѧ����ʽ��
��3�������ж��¶Ȳ����Ч�ԣ�Ȼ������¶Ȳ�ƽ��ֵ��
���ȸ���Q=m?c?��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-
| Q |
| n |
��a��װ�ñ��¡�����Ч�����õ�����ƫС��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���õ�����ƫС��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ�
��2����������Ҫ�ڳ���ƿ����С�ձ��г������������������������õ���������ƽ���ձ���ҩ�ף��ʴ�Ϊ���٢ڢݣ�
��1���к��ȵ�ʵ��Ĺؼ��DZ��£���������ĭ���ϵ������Ƿ�ֹ�кͷ�Ӧʱ������ʧ���ʴ�Ϊ����ֹ�кͷ�Ӧʱ������ʧ��
��2��ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������ϡ�������������ϡ��Һ��Ӧ���Ȼ�ѧ����ʽΪ��
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��
| 1 |
| 2 |
| 1 |
| 2 |
��3����4���¶Ȳ�ֱ�Ϊ��4.2�棬3.8�棬3.9�棬4.1�棬4�����ݶ���Ч���¶Ȳ�ƽ��ֵ=
| �Ĵ�ʵ����(��ֹ�¶�-��ʼ�¶�)�ĺ� |
| 4 |
| 4.2��+3.8��+3.9��+4.1�� |
| 4 |
�ʴ�Ϊ��4.0��
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m?c?��T=80g��4.18J/��g?�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-
| 1.3376KJ |
| 0.025mol |
�ʴ�Ϊ��-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b����
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���c��ȷ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����d��ȷ��
�ʴ�Ϊ��acd��

ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��
������0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH���� g��
��2����ͼ1��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ���� ��
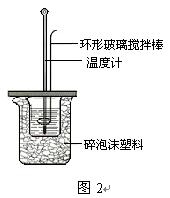
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ2��ʾ��
��3��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ/mol����
��4��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�ڽ�����Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c=4.18 J/(g����)�����к��ȡ�H= ��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ�� ��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L H2SO4��Һ�����к��ȵIJⶨ��
��.����0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ����������Ҫ����NaOH���� g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(�����) ��
|
���� |
������ƽ(������) |
С�ձ� |
����ǯ |
������ |
ҩ�� |
��Ͳ |
|
���� |
|
|
|
|
|
|
|
��� |
a |
b |
c |
d |
e |
f |
��.�ⶨ�к��ȵ�ʵ��װ����ͼ��ʾ

��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3 kJ��mol��1)��_______________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL�������ʵ�飬ʵ���������±���
������д�±��еĿհף�
|
�¶� ʵ����� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
ƽ���¶Ȳ� (t2��t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
30.1 |
|
|
2 |
27.0 |
27.4 |
27.2 |
33.3 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
�ڸ�������ʵ�����ݼ�������к���Ϊ53.5 kJ/mol�������к��ȵ�����ֵ57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)______��
a��ʵ��װ�ñ��¡�����Ч����
b������ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
ijʵ��С����0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ�����к��ȵIJⶨ��
������0.50 mol��L-1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH�������� ��g��
��2������ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ������ ������
|
���� |
������ƽ (������) |
С�ձ� |
����ǯ |
������ |
ҩ�� |
��Ͳ |
|
���� |
|
|
|
|
|
|
|
��� |
a |
b |
c |
d |
e |
f |
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
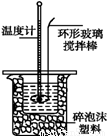
��1��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ�����к���Ϊ57.3 kJ��mol-1�� ���� ���� ���� ���� ��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
|
�¶� ʵ������� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
�¶Ȳ� ƽ��ֵ (t2-t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
29.6 |
|
|
2 |
27.0 |
27.4 |
27.2 |
31.2 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
|
���ϱ��е��¶Ȳ�ƽ��ֵΪ�� ��
�ڽ�����Ϊ0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ���ܶȶ���1 g��cm-3���кͺ�������Һ�ı�����c=4.18 J��(g����)-1�����к��Ȧ�H=������ ��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ��mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ������������
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�