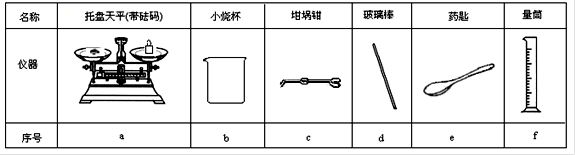
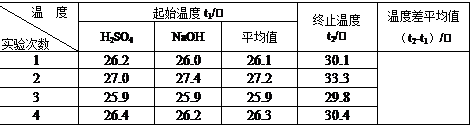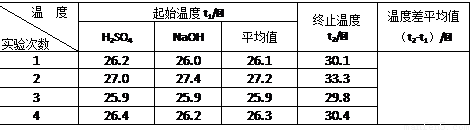��Ŀ����
ijʵ��С����0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ�����к��ȵIJⶨ��
������0.50 mol��L-1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH�������� ��g��
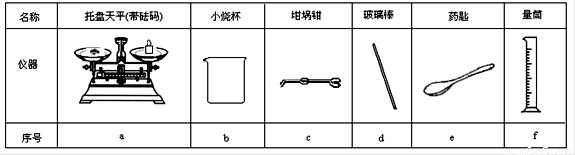
��2������ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ������ ������
|
���� |
������ƽ (������) |
С�ձ� |
����ǯ |
������ |
ҩ�� |
��Ͳ |
|
���� |
|
|
|
|
|
|
|
��� |
a |
b |
c |
d |
e |
f |
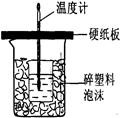
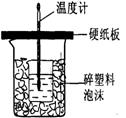
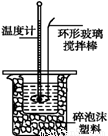
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ�����к���Ϊ57.3 kJ��mol-1�� ���� ���� ���� ���� ��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
|
�¶� ʵ������� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
�¶Ȳ� ƽ��ֵ (t2-t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
29.6 |
|
|
2 |
27.0 |
27.4 |
27.2 |
31.2 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
|
���ϱ��е��¶Ȳ�ƽ��ֵΪ�� ��
�ڽ�����Ϊ0.50 mol��L-1 NaOH��Һ��0.50 mol��L-1������Һ���ܶȶ���1 g��cm-3���кͺ�������Һ�ı�����c=4.18 J��(g����)-1�����к��Ȧ�H=������ ��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ��mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ������������
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��.��1��5.0��1�֣�
��2��a��b��e��2�֣�
��.��1��1/2H2SO4(aq)+
NaOH(aq) 1/2Na2SO4(aq)+ H2O(l)����H=-57.3 kJ��mol-1��2�֣�
1/2Na2SO4(aq)+ H2O(l)����H=-57.3 kJ��mol-1��2�֣�
��2����4.0��2�֣��� ��-53.5 kJ��mol-1��2�֣�����a��c��d��2�֣�
��������
�����������.��1����Ҫ����NaOH����m=nM=cVM=0.5mol/L��0.5L��40g/mol=5.0g��
��2����������Ҫ�ڳ���ƿ����С�ձ��г������������������������õ���������ƽ���ձ���ҩ�ף��ʴ�Ϊ��a b e ��
��.��1���к���Ϊ����к�����1mol H2Oʱ���ʱ䣬���Ա�ʾϡ�����ϡ���������к��ȵ��Ȼ�ѧ����ʽΪ��1/2H2SO4(aq)+ NaOH(aq)=1/2Na2SO4(aq)+ H2O(l)����H=-57.3 kJ��mol-1
��2���ٵ�2������������������ɾ������������������ƽ��ֵ���¶Ȳ�ƽ��ֵ=��(30.1−26.1)+(29.8−25.9)+(30.4−26.3)����3=4.0��C��
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m•c•��T=80g��4.18J/��g•�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-1.3376kJ��0.025mol=--53.5 kJ��mol-1��
��a��ʵ��װ�ñ��¡�����Ч������ã�����Ӱ��ʵ��������ȷ��b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ������57.3kJ/mol������c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���ȷ��d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�Ҫ���¶ȼƻ���ֱ�ٲⶨH2SO4��Һ���¶ȣ���ȷ��
���㣺���⿼����Һ�����ơ��Ȼ�ѧ����ʽ����д����Ӧ�ȵļ��㡢��������

ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L������Һ�����к��ȵIJⶨ��
������0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH���� g��
��2����ͼ1��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ���� ��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ2��ʾ��
��3��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ/mol����
��4��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�ڽ�����Ϊ0.50 mol/L NaOH��Һ��0.50 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c=4.18 J/(g����)�����к��ȡ�H= ��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ�� ��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
ijʵ��С����0.50 mol/L NaOH��Һ��0.50 mol/L H2SO4��Һ�����к��ȵIJⶨ��
��.����0.50 mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ����������Ҫ����NaOH���� g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(�����) ��
|
���� |
������ƽ(������) |
С�ձ� |
����ǯ |
������ |
ҩ�� |
��Ͳ |
|
���� |
|
|
|
|
|
|
|
��� |
a |
b |
c |
d |
e |
f |
��.�ⶨ�к��ȵ�ʵ��װ����ͼ��ʾ
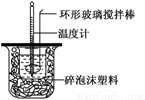
��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ57.3 kJ��mol��1)��_______________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL�������ʵ�飬ʵ���������±���
������д�±��еĿհף�
|
�¶� ʵ����� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
ƽ���¶Ȳ� (t2��t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
30.1 |
|
|
2 |
27.0 |
27.4 |
27.2 |
33.3 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
�ڸ�������ʵ�����ݼ�������к���Ϊ53.5 kJ/mol�������к��ȵ�����ֵ57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)______��
a��ʵ��װ�ñ��¡�����Ч����
b������ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ��ϡ�����С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�