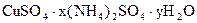��Ŀ����
��14�֣���ҵ�Ͽ������÷����ᡢ̼������Ʊ�����ʯ��Na3AlF6�����乤�����£�
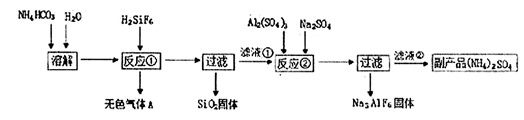
��ش��������⣺
��1����Ӧ�������ɵ���ɫ����A�� ����Һ�ٵ������� ��
��2�����ⶨNH4HCO3��Һ�����ԣ������ԭ�������ӷ���ʽ�����ֱ���˵�����ɣ�
��
��3������Ӧ�ں��˵õ��ı�����Na3AlF6�����壬�ù����ϸ��������ʣ���Ҫϴ�ӣ���������֤������ʯ�����Ѿ�ϴ�Ӹɾ� ��
��4���¶Ⱥ�NH4HCO3Ũ�Ȼ�Ӱ�췴Ӧ�ٵ����ʡ����������ʵ�鷽��̽���¶Ⱥ�NH4HCO3Ũ�ȶԷ�Ӧ�����ʵ�Ӱ�졣���ڱ���հ״���д�ʵ��ķ�Ӧ������
��5����ͼ����Һ�����ʵ������������¶ȱ仯������ͼ��
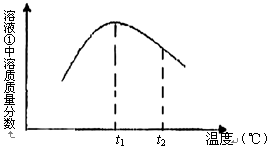

�����t2��ʱ����Һ�������ʵ����������½���ԭ���� ��

��ش��������⣺
��1����Ӧ�������ɵ���ɫ����A�� ����Һ�ٵ������� ��
��2�����ⶨNH4HCO3��Һ�����ԣ������ԭ�������ӷ���ʽ�����ֱ���˵�����ɣ�
��
��3������Ӧ�ں��˵õ��ı�����Na3AlF6�����壬�ù����ϸ��������ʣ���Ҫϴ�ӣ���������֤������ʯ�����Ѿ�ϴ�Ӹɾ� ��
��4���¶Ⱥ�NH4HCO3Ũ�Ȼ�Ӱ�췴Ӧ�ٵ����ʡ����������ʵ�鷽��̽���¶Ⱥ�NH4HCO3Ũ�ȶԷ�Ӧ�����ʵ�Ӱ�졣���ڱ���հ״���д�ʵ��ķ�Ӧ������
| ʵ���� | ʵ��Ŀ�� | �¶� | c��NH4HCO3�� |
| I | Ϊ����ʵ�������� | 60�� | c1 |
| II | ̽��Ũ�ȶԷ�Ӧ�����ʵ�Ӱ�� | | c2 |
| III | ̽���¶ȶԷ�Ӧ�����ʵ�Ӱ�� | 80�� | |


�����t2��ʱ����Һ�������ʵ����������½���ԭ���� ��
��1��CO2 NH4F
��2��NH
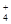 +H2O
+H2O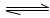 NH3��H2KO+H+
NH3��H2KO+H+HCO
 +H2O
+H2O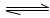 H2CO3+OH
H2CO3+OH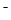
NH
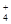 ��HCO
��HCO ��ˮ��̶Ȼ�����ͬ
��ˮ��̶Ȼ�����ͬ��3��ȡϴ�Ӻ����Һ�������еμ�BaCl2��Һ��������˵���Ѿ�ϴ�Ӹɾ������а�ɫ�������ɣ�˵��û��ϴ�Ӹɾ��������������������֣�
��4��60�� c1
��5���¶�����NH4F��ֽ⣨����NH4HCO3��ֽ�Ҳ���֣�
��1��̼�������ܺͷ����ᷴӦ���ɶ�����̼���塢�������衢������Լ�ˮ���������費����ˮ�������������ˮ��
��2��NH4+��HCO3-��ˮ�⣬ǰ��ˮ�������ԣ�����ˮ���Լ��ԣ�
��3������Ӧ�ں��˵õ��ı�����Na3AlF6�������ϸ�������������泥�����������ļ��鷽��ȷ��������Ĵ��ڣ�
��4���Ա�ʵ��Ĺؼ��ǿ��Ʊ����������¶�һ��ʱ���仯Ũ�ȣ���Ũ��һ��ʱ���仯�¶ȣ�
��5������淋����ʺ��Ȼ�����ƣ����ȶ��������ֽ⣮
�⣺��1��̼�������ܺͷ����ᷴӦ���ɶ�����̼���塢�������衢������Լ�ˮ���������費����ˮ�����˺��γ������������������ˮ���γ���Һ���ʴ�Ϊ��CO2��NH4F��
��2��NH4+��HCO3-��ˮ�⣬ǰ��ˮ�������ԣ�����ˮ���Լ��ԣ�����ˮ��̶���ͬ����Һ��������Ũ�Ⱥ�����������Ũ����ȣ���Һ�����ԣ��ʴ�Ϊ��NH4++H2O NH3?H2O+H+��HCO3-+H2O
NH3?H2O+H+��HCO3-+H2O H2CO3+OH-��NH4+��HCO3-��ˮ��̶Ȼ�����ͬ��
H2CO3+OH-��NH4+��HCO3-��ˮ��̶Ȼ�����ͬ��
��3������Ӧ�ں��˵õ��ı�����Na3AlF6�������ϸ�������������泥�������ļ��鷽�������������Ӳ�������������İ�ɫ��������ȷ��������Ĵ��ڣ�ֻҪ��������ڣ�������δϴ�Ӹɾ����ʴ�Ϊ��ȡϴ�Ӻ����Һ�������еμ�BaCl2��Һ��������˵���Ѿ�ϴ�Ӹɾ������а�ɫ�������ɣ�˵��û��ϴ�Ӹɾ���
��4���Ա�ʵ��Ĺؼ��ǿ��Ʊ����������¶�һ��ʱ��ͨ���仯Ũ��������Ũ�ȵ�Ӱ�죬�ʢ�͢���¶���ȣ���Ũ��һ��ʱ��ͨ���仯�¶��������¶ȵ�Ӱ�죬�ʢ�͢��¶Ȳ�ͬ����Ũ��Ҫ��ͬ���ʴ�Ϊ��60�棻 c1��
��5������淋����ʺ��Ȼ�����ƣ����ȶ��������ֽ⣨5���¶�����NH4F��ֽ⣬�ʴ�Ϊ���¶�����NH4F��ֽ⣮
��2��NH4+��HCO3-��ˮ�⣬ǰ��ˮ�������ԣ�����ˮ���Լ��ԣ�
��3������Ӧ�ں��˵õ��ı�����Na3AlF6�������ϸ�������������泥�����������ļ��鷽��ȷ��������Ĵ��ڣ�
��4���Ա�ʵ��Ĺؼ��ǿ��Ʊ����������¶�һ��ʱ���仯Ũ�ȣ���Ũ��һ��ʱ���仯�¶ȣ�
��5������淋����ʺ��Ȼ�����ƣ����ȶ��������ֽ⣮
�⣺��1��̼�������ܺͷ����ᷴӦ���ɶ�����̼���塢�������衢������Լ�ˮ���������費����ˮ�����˺��γ������������������ˮ���γ���Һ���ʴ�Ϊ��CO2��NH4F��
��2��NH4+��HCO3-��ˮ�⣬ǰ��ˮ�������ԣ�����ˮ���Լ��ԣ�����ˮ��̶���ͬ����Һ��������Ũ�Ⱥ�����������Ũ����ȣ���Һ�����ԣ��ʴ�Ϊ��NH4++H2O
 NH3?H2O+H+��HCO3-+H2O
NH3?H2O+H+��HCO3-+H2O H2CO3+OH-��NH4+��HCO3-��ˮ��̶Ȼ�����ͬ��
H2CO3+OH-��NH4+��HCO3-��ˮ��̶Ȼ�����ͬ����3������Ӧ�ں��˵õ��ı�����Na3AlF6�������ϸ�������������泥�������ļ��鷽�������������Ӳ�������������İ�ɫ��������ȷ��������Ĵ��ڣ�ֻҪ��������ڣ�������δϴ�Ӹɾ����ʴ�Ϊ��ȡϴ�Ӻ����Һ�������еμ�BaCl2��Һ��������˵���Ѿ�ϴ�Ӹɾ������а�ɫ�������ɣ�˵��û��ϴ�Ӹɾ���
��4���Ա�ʵ��Ĺؼ��ǿ��Ʊ����������¶�һ��ʱ��ͨ���仯Ũ��������Ũ�ȵ�Ӱ�죬�ʢ�͢���¶���ȣ���Ũ��һ��ʱ��ͨ���仯�¶��������¶ȵ�Ӱ�죬�ʢ�͢��¶Ȳ�ͬ����Ũ��Ҫ��ͬ���ʴ�Ϊ��60�棻 c1��
��5������淋����ʺ��Ȼ�����ƣ����ȶ��������ֽ⣨5���¶�����NH4F��ֽ⣬�ʴ�Ϊ���¶�����NH4F��ֽ⣮

��ϰ��ϵ�д�
 �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ