��Ŀ����
����Ŀ�����ú�̼������ϳ���ȼ���Ƿ�չ��̼���õ���Ҫ��������֪CO(g)+2H2(g) ![]() CH3OH(g)���������������ͼ��ʾ�����������������ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ������ ��
CH3OH(g)���������������ͼ��ʾ�����������������ֱ��ʾ��ʹ�ô�����ʹ�ô�������������������ж���ȷ������ ��
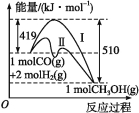
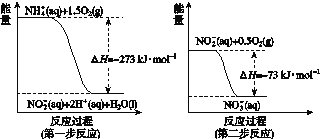
A���÷�Ӧ�Ħ�H=+91 kJ��mol-1
B������������÷�Ӧ�Ħ�H��С
C����Ӧ��������������������������
D������÷�Ӧ����Һ̬CH3OH����H����
���𰸡�C
��������A����Ӧ���������������������������÷�Ӧ���ȣ���A����B�������������Ӧ�Ȳ��䣬��B����C����ͼ���֪����Ӧ����������������������������Ϊ���ȷ�Ӧ����C��ȷ��D������÷�Ӧ����Һ̬CH3OH���ų��������������Ӧ��Ϊ��ֵ������H��С����D����ѡC��

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�����Ŀ��ij��ѧ��Ӧ2A![]() B + D�����ֲ�ͬ�����½��У�B��D����ʼŨ��Ϊ0����Ӧ��A��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±���ʾ�������¶�Ϊ���϶ȣ��棩��
B + D�����ֲ�ͬ�����½��У�B��D����ʼŨ��Ϊ0����Ӧ��A��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±���ʾ�������¶�Ϊ���϶ȣ��棩��
ʵ�� ��� | Ũ�� ʱ�� �¶� | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
1 | 800 | 1. 0 | 0.80 | 0.67 | 0.57 | 0.50 | c1 | 0.50 |
2 | 800 | 1.0 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
3 | T3 | 1.0 | 0.40 | 0.25 | 0.20 | 0.20 | 0.20 | 0.20 |
���������ݣ��ش��������� ��
��1����ʵ��1�У��Է�Ӧ��AŨ�ȵı仯��ʾ�÷�Ӧ��0~10min�ڵ�ƽ����Ӧ����Ϊ________ ��
��2����ʵ��1�У�c1��ֵΪ ________ ��
��3����ʵ��1��2����һ��ʵ��ʹ�������������ж���ʵ�� ________ ��ѡ�1����2����ʹ������������
��4����ʵ��1��3�з�Ӧ�¶Ȳ�ͬ���ж�T3 ________ 800��ѡ���������������