��Ŀ����
1����ѧ�о�����������Cu2O����Ϊ̫����ֽ�ˮ�Ĵ�����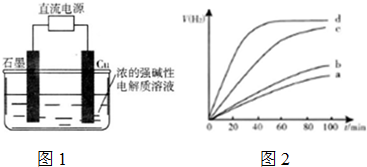
I��������ȡCu2O�ķ�����
a����̿���ڸ��������»�ԭCuO�Ʊ�Cu2O��
b���������ǻ�ԭ���Ƶ�Cu��OH��2�Ʊ�Cu2O��
c����ⷨ�Ʊ�Cu2O��װ����ͼ1��
d��ʪ��ѧ����������������Һ̬�£�N2H4����ԭ����Cu��OH��2
���Ʊ�����Cu2O��ͬʱ�ų�N2��
��1������cͨ�����������Ƶ�Cu2O��д�������ĵ缫��Ӧʽ2Cu+2OH--2e-=Cu2O+H2O��
��2������d�Ļ�ѧ����ʽΪN2H4+4Cu��OH��2=2Cu2O+N2��+6H2O����������Cu2O�����Ӵ�СԼ��ʮ���ף��Ѿ����ɵ�ʵ�鷽���Ƕ����ЧӦ��
��3������d�õ��IJ�Ʒ�г�����Cu2O��ȡij��Ʒ3.52g������Cu2O��Cu��������������ϡ���ᣬ��ַ�Ӧ��õ���״���µ�NO����448mL�����Ʒ��Cu2O����������Ϊ81.8%��
�����Ƶõ�Cu2O���д��ֽ�ˮ��ʵ��
��4��һ���¶��£���2L�ܱ������м�������Cu2O��ͨ��0.10molˮ������������Ӧ��
2H2O��g��$?_{Cu_{2}O}^{����}$2H2��g��+O2��g����H�T+484KJ•mol-1
��ͬʱ�β���O2�����ʵ������±���
| ʱ��/min | 20 | 40 | 60 | 80 |
| N��O2��/mol | 0.0010 | 0.0016 | 0.0020 | 0.0020 |
��5�����������ַ����Ƶõ�Cu2O��ij��ͬ�����·ֱ��ˮ���ֽ⣬�������������V��H2����ʱ��t�仯��ͼ2��
����������ȷ����ACD������ĸ���ţ���
A��c��d�����Ƶõ�Cu2O��Ч����Խϸ�
B��d�����Ƶõ�Cu2O������ʱ��ˮ��ƽ��ת�������
C����Ч����Cu2O�����Ĵ�ϸ��������Ե��й�
D��Cu2O��ˮ�ֽ�ʱ����Ҫ���˵��¶ȣ�
���� I����1�����ʱCu������ʧ��������Cu2O��
��2�����������£�N2H4������Cu��OH��2��Ӧ����Cu2O��N2��ˮ������Cu2O��ˮ���ܷ��������ЧӦ��
��3���躬ͭ�����ʵ���Ϊxmol��������ͭ�����ʵ���Ϊymol������������ϵ�͵����غ���ʽ�����ͭ�����ʵ������ټ����ͭ������������
��4�����ݻ�ѧ��Ӧ����֮�ȵ��ڼ�����֮�ȼ��㷴Ӧ���ʣ���ƽ��ʱ����������0.002mol�������Ȼ�ѧ����ʽ���㣻
��5������ͼ�����ߵı仯�����жϣ�����б��Խ��Ӧ����Խ���¶����ߣ�б����С���Դ˽��
��� �⣺I����1�����ʱCu������ʧ��������Cu2O���缫����ʽΪ2Cu+2OH--2e-=Cu2O+H2O���ʴ�Ϊ��2Cu+2OH--2e-=Cu2O+H2O��
��2�����������£�N2H4������Cu��OH��2��Ӧ����Cu2O��N2��ˮ���䷴Ӧ�Ļ�ѧ����ʽΪ��N2H4+4Cu��OH��2=2Cu2O+N2��+6H2O������Cu2O��ˮ��Һ���γɽ��壬���Ը�����Һ�Ƿ���ж����ЧӦ�������Ƿ���������Cu2O��
�ʴ�Ϊ��N2H4+4Cu��OH��2=2Cu2O+N2��+6H2O�������ЧӦ��
��3����״���µ�NO����148mL����NO�����ʵ���Ϊ��$\frac{0.448L}{22.4L/mol}$=0.02mol
�躬ͭ�����ʵ���Ϊxmol��������ͭ�����ʵ���Ϊymol��
64x+144y=3.52g��
2x+2y=0.02��3��
���x=0.01mol��y=0.02mol��
w��Cu2O��=$\frac{0.02mol��144g/mol}{3.52g}$��100%=81.8%��
�ʴ�Ϊ��81.8%��
��4���ɱ������ݿ�֪ǰ20minʱv��O2��=$\frac{\frac{0.0010mol}{2L}}{20min}$=2.5��10-5 mol��L-1��min -1��
��v��H2O��=2v��O2��=5.0��10-5 mol��L-1��min -1����ƽ��ʱ����������0.002mol��������Ҫ���յĹ���Ϊ0.002mol��484kJ•mol-1=0.968kJ��
�ʴ�Ϊ��5.0��10-5 mol��L-1��min -1��0.968��
��5��A��c��d����б�ʽϴ�˵����Ӧ���ʽϴ���c��d�����Ƶõ�Cu2O��Ч����Խϸߣ���A��ȷ��
B������ֻ�ı䷴Ӧ���ʣ���Ӱ��ƽ���ƶ�����B����
C���ò�ͬ�ķ����Ʊ���������ͭ�Ŀ�����С��ͬ����ͼ���֪��Ч����ͬ�����Ч����Cu2O�����Ĵ�ϸ��������Ե��йأ���C��ȷ��
D���¶����ߣ�б����С��˵��Cu2O��ˮ�ֽ�ʱ����Ҫ���˵��¶ȣ���D��ȷ��
�ʴ�Ϊ��ACD��
���� ���⿼���Ϊ�ۺϣ��漰������ͭ���Ʊ�����Ӧ���ʵļ��㡢��ѧƽ���ƶ������ԭ����Ӧ�õȣ�������ѧ���ķ����������Ի���֪ʶ��Ӧ�������ͼ��������Ŀ��飬ע����յ缫����ʽ����д���Ѷ��еȣ�

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�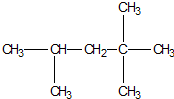 ��Ϊ��������ȼ�ϵ����ͣ�����Ҫ�ɷ��������飬��ṹ��ʽ��ͼ���и�ʽ���Կ϶������������黥��Ϊͬϵ����ǣ�������
��Ϊ��������ȼ�ϵ����ͣ�����Ҫ�ɷ��������飬��ṹ��ʽ��ͼ���и�ʽ���Կ϶������������黥��Ϊͬϵ����ǣ�������| A�� | C2H4 | B�� | C8H18 | C�� | C7H16 | D�� | C6H12 |
| ���� | MgCl2 | Mg��OH��2 | MgCO3 |
| �ܽ�ȣ�g/100g�� | 74 | 0.00084 | 0.01 |
| A�� | ��֪MgCO3��K=6.82��10-6moL-2•L-2�������й���MgCO3����Һ�У�����c��Mg2+��=c��CO32-������c��Mg2+��•c��CO32-��=6.82��10-6moL2-•L-2 | |
| B�� | ����������������ˮ��ϣ����ȡ����գ����յĹ��������ͬ | |
| C�� | ��ȥ�����к��е�MgCl2���ʣ���ѳ����Լ�ΪNa2CO3 | |
| D�� | ��ʯ��ˮ��������Mg2+��HCO3��Ӳˮ�����������ӷ�Ӧ�ķ���ʽΪMg2++2HCO3+Ca2+2OH�TCaCO3��+MgCO3��+2H2O |
| A�� | ԭ������ | B�� | ���ʵ��� | C�� | �������� | D�� | ���� |
 ʵ��Ŀ�ĵ���
ʵ��Ŀ�ĵ���
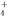 ��Al3����AlO
��Al3����AlO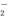 ��SO
��SO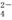 ��SiO
��SiO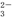 ��CO
��CO �������ǣ��϶������ڵ������ǡ�
�������ǣ��϶������ڵ������ǡ�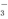 ��3H2O��3I2��6OH��
��3H2O��3I2��6OH�� ��OH��?NH3����H2O
��OH��?NH3����H2O ��Ag����NO����H2O
��Ag����NO����H2O
 NH3��H2O
NH3��H2O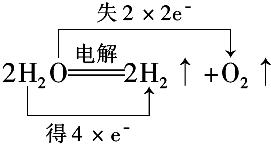 ��
��