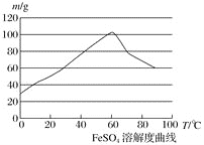
��Ŀ����
����Ŀ��ijѧϰ��ȤС��Ϊ�ⶨij���Ѿ���SO2�ĺ������������ͼ��ʾ��װ�ã�
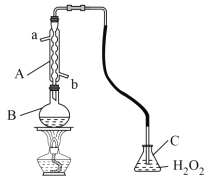
ʵ�鲽�����£���B�м���300.00mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C��H2O2��ȫ��Ӧ����ȥC��ʣ���H2O2����0.0900mol��L-1NaOH��Һ�ⶨ��Ӧ���ɵ��ᣬ����NaOH��Һ25.00mL��
��1��C��ͨ��SO2������Ӧ�Ļ�ѧ����ʽΪ___��
��2��ͨ��������������Ѿ���SO2�ĺ���(��g��L-1Ϊ��λ����ÿ�����Ѿ��к���SO2������) ___��(д���������)
��3���òⶨ�����ʵ��ֵƫ�ߣ����ܵ�ԭ����____����Դ�ԭ�����һ���Ľ���ʩ��___��
���𰸡�SO2+H2O2=H2SO4 0.24 g/L �����ӷ������Ȼ������װ��C�� B���ò��ӷ���ǿ���������������
��������
(1)����������л�ԭ�ԣ��ܹ���˫��ˮ��Ӧ�������ᣬ�ݴ�д����Ӧ�Ļ�ѧ����ʽ��
(2)���ݹ�ϵʽ2NaOH��H2SO4��SO2���������Ƶ����ʵ������������������������ټ���������Ѿ��еĶ�����������
(3)�����ǻӷ����ᣬ�ӷ����������������ƣ��ݴ˷������
(1)˫��ˮ���������ԣ��ܹ��������������������ᣬ��Ӧ�Ļ�ѧ����ʽΪ��SO2+H2O2=H2SO4���ʴ�Ϊ��SO2+H2O2=H2SO4��
(2)����2NaOH��H2SO4��SO2��֪SO2������Ϊ��![]() ��(0.0900mol/L��0.025L)��64g/mol=0.072g�������Ѿ��еĶ���������Ϊ��
��(0.0900mol/L��0.025L)��64g/mol=0.072g�������Ѿ��еĶ���������Ϊ��![]() =0.24g/L���ʴ�Ϊ��0.24 g/L��
=0.24g/L���ʴ�Ϊ��0.24 g/L��
(3)���������ǻӷ����ᣬ�ӷ������ܹ������������ƣ�ʹ�����ĵ�����������Һ������ⶨ���ƫ�ߣ���˿����ò��ӷ���ǿ�ᣬ������������ᣬ�ʴ�Ϊ�������ӷ������Ȼ������װ��C�У�B���ò��ӷ���ǿ��������������ᡣ

����Ŀ��ij��ȤС���ͬѧ��һ��������Ʒ(�������ʣ����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ)���з������ס��ҡ�����λͬѧ�ֱ����ʵ�飬����һλͬѧ���õ�ϡ����������Ʒǡ����ȫ��Ӧ��ʵ���������±���
�� | �� | �� | |
�ձ�+ϡ���������/g | 200 | 150 | 150 |
����������Ʒ������/g | 9 | 9 | 14 |
��ַ�Ӧ���ձ�+ʣ���������/g | 208.7 | 158.7 | 163.7 |
��������������ݣ��ش��������⣺
��1����λͬѧ���õ�ϡ������������Ʒǡ����ȫ��Ӧ_____��
��2������������Ʒ��������������_____��