��Ŀ����
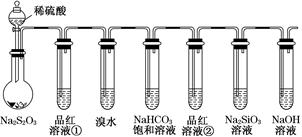
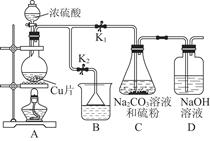
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������__________________�����з�����Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ʵ������У�װ��B��C�в���������ֱ���___________��___________����Щ����ֱ�˵��SO2���е�������___________��___________��װ��B�з�����Ӧ�����ӷ���ʽΪ_________________________________________________��
��3��װ��D��Ŀ����̽��SO2��Ʒ����Һ���õĿ����ԣ���д��ʵ�����������____________��
��4��β���ɲ���______________��Һ���ա�

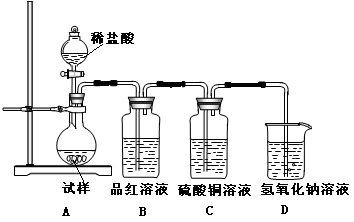
��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������__________________�����з�����Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ʵ������У�װ��B��C�в���������ֱ���___________��___________����Щ����ֱ�˵��SO2���е�������___________��___________��װ��B�з�����Ӧ�����ӷ���ʽΪ_________________________________________________��
��3��װ��D��Ŀ����̽��SO2��Ʒ����Һ���õĿ����ԣ���д��ʵ�����������____________��
��4��β���ɲ���______________��Һ���ա�
��1��������ƿ
Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O
��2����Һ���Ϻ�ɫ��Ϊ��ɫ ��ɫ��Һ�г��ֻ�ɫ���� ��ԭ�� ������
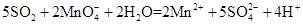
��3��Ʒ����Һ��ɫ�رշ�Һ©������������ȼ�ƾ��Ƽ��ȣ�Ʒ����Һ�ָ�Ϊ��ɫ
��4��NaOH���𰸺������ɣ�
Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O
��2����Һ���Ϻ�ɫ��Ϊ��ɫ ��ɫ��Һ�г��ֻ�ɫ���� ��ԭ�� ������
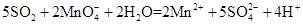
��3��Ʒ����Һ��ɫ�رշ�Һ©������������ȼ�ƾ��Ƽ��ȣ�Ʒ����Һ�ָ�Ϊ��ɫ
��4��NaOH���𰸺������ɣ�
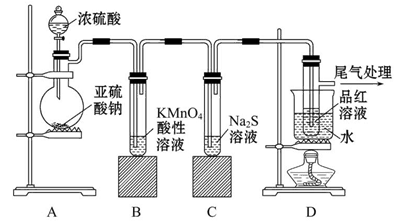
���⿼����SO2���Ʊ������ʡ���ѧʵ��Ļ�����������1����������ƿ�У�Բ����ƿ��ƽ����ƿ��������ƿ��ͼʾ�д�֧�ܵ�Ϊ������ƿ����2��SO2���л�ԭ�ԣ���ʹ���Ը��������Һ��ɫ�����ӷ���ʽΪ��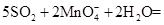
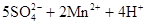 ��SO2����-2�۵������ӷ�Ӧ��������������������ԡ���3��SO2��Ʒ����Һ���õĿ�����ָ��SO2ʹƷ����Һ��ɫ�����Ⱥ�Ʒ����Һ�ָֻ���ɫ��ע��ʵ�������Ʒ����Һ��ɫ��Ҫ�رշ�Һ©������������4��SO2Ϊ�������壬һ���ü�����Һ���գ�Ҳ�������Ը��������Һ��ǿ��������Һ���ա�
��SO2����-2�۵������ӷ�Ӧ��������������������ԡ���3��SO2��Ʒ����Һ���õĿ�����ָ��SO2ʹƷ����Һ��ɫ�����Ⱥ�Ʒ����Һ�ָֻ���ɫ��ע��ʵ�������Ʒ����Һ��ɫ��Ҫ�رշ�Һ©������������4��SO2Ϊ�������壬һ���ü�����Һ���գ�Ҳ�������Ը��������Һ��ǿ��������Һ���ա�
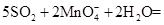
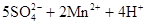 ��SO2����-2�۵������ӷ�Ӧ��������������������ԡ���3��SO2��Ʒ����Һ���õĿ�����ָ��SO2ʹƷ����Һ��ɫ�����Ⱥ�Ʒ����Һ�ָֻ���ɫ��ע��ʵ�������Ʒ����Һ��ɫ��Ҫ�رշ�Һ©������������4��SO2Ϊ�������壬һ���ü�����Һ���գ�Ҳ�������Ը��������Һ��ǿ��������Һ���ա�
��SO2����-2�۵������ӷ�Ӧ��������������������ԡ���3��SO2��Ʒ����Һ���õĿ�����ָ��SO2ʹƷ����Һ��ɫ�����Ⱥ�Ʒ����Һ�ָֻ���ɫ��ע��ʵ�������Ʒ����Һ��ɫ��Ҫ�رշ�Һ©������������4��SO2Ϊ�������壬һ���ü�����Һ���գ�Ҳ�������Ը��������Һ��ǿ��������Һ���ա�
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ