��Ŀ����
����Ŀ��ˮú������Ҫ�ɷ���������һ����̼����ˮ�����ͳ��ȵ�����ú��̿���ö���.����Ҫ�Ļ���ԭ�ϣ������ںϳɼ״��ͼ��ѵ��л��������֪��
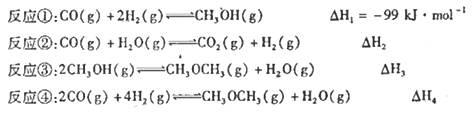
(1)��H1= ______(�á�H2����H3����H4����
(2)��Ӧ�ڵĴ��ڣ��ɴ�����CH30CH3�IJ��ʣ�ԭ����: ________��
C0(g)��H2(g)�����ʵ���֮��1:2��ϣ�һ����������1L�̶��ݻ��ڷ�����Ӧ�٣���ͼ��ʾ�¶ȷֱ�Ϊ300�桢500����ܱ������У��״������ʵ�����ʱ��Ĺ�ϵ���ش��������⣺

��C��D����ƽ�ⳣ��KC _____KD(��>��<��= )��
���������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2�����йظ���ϵ��˵����ȷ���� _______��
a. ����Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
b.�״������ʵ�������
c.����ƽ��ʱn(H2)/n(CH3OH)����
(4)�¶�ΪTʱ�����ݻ�1L���ܱ������ס����зֱ����һ������CO(g)��H20(g),������Ӧ�ڣ�����������£�

�ټ������У���Ӧ��t1min�ڵ�ƽ������v(H2)= ____mol/(Lmin)��
����������a=_____mol��
�۽��ͽ����¶�ʹC02ƽ��Ũ�������ԭ��___________��
���𰸡� ����H4-��H3 ��/2 �˷�Ӧ������H2O(g)ͬʱ������H2���������ڷ�Ӧ�������ƶ� > ab 0.4/t1 1.6 �÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ���CO2ƽ��Ũ������
����������1�����ݸ�˹���ɿ�֪����������/2���ɵõ���H1������H4-��H3 ��/2����2����Ӧ��������H2O(g)ͬʱ������H2���������ڷ�Ӧ�������ƶ������Է�Ӧ�ڵĴ��ڣ��ɴ�����CH30CH3�IJ��ʡ���3���ٷ�Ӧ���Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ����С�ᣬ��C��D����ƽ�ⳣ��KC��KD����a.�Դ���E�����ϵ���ѹ����ԭ����1/2������ѹǿ������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ죬a��ȷ��b.����Ӧ�����С������ѹǿƽ��������Ӧ������У��״������ʵ������ӣ�b��ȷ��c.����ƽ��ʱn(H2)/n(CH3OH)��С��c����ѡab����4���ټ������У���Ӧ��t1min ������CO�����ʵ���Ϊ��1.2mol-0.8mol=0.4mol�����ʱ�������CO��ʾ��ƽ����Ӧ����Ϊ��v��CO����0.4mol/(1L��t1min)��0.4/t1mol/��Lmin�������ݻ�ѧ�������뷴Ӧ���ʳ����ȿ�֪��v��H2����v��CO����0.4/t1mol/��Lmin������CO��g��+H2O��g��CO2��g��+H2��g��Ϊ�����������ķ�Ӧ��ѹǿ��Ӱ�컯ѧƽ�⣬��ס��һ�Ϊ��Чƽ�⣬�ﵽƽ��ʱ��Ӧ��ת������ȣ���0.8/1.2=a/2.4����ã�a=1.6���۷�ӦCO��g��+H2O��g��CO2��g��+H2��g���ʱ�С��0��Ϊ���ȷ�Ӧ�������¶Ⱥ�ƽ�����������ƶ������¶�����̼��Ũ������

