��Ŀ����
ij������Һ�к��б��ӡ����ᣬʵ��С��Ը÷�Һ����̽����������·�����
��֪�۵㣺����16.6�桢����43�档�е㣺����118�桢����182�档
��1��д���ڵķ�Ӧ��ѧ����ʽ ��
��2�������B�IJ��������� ��
��3���ֶ�����C�����ʽ���ʵ��̽�����������ʵ��С�鰴Ҫ�����ʵ����̼�¼���ڴ������д��ʵ�������Ԥ�������������͡�
��ѡ�Լ�������ˮ��ϡHNO3��2moL��L��1NaOH��0.1 mol ?L��1KSCN������KMnO4�� Һ��FeCl3��Һ��������ˮ����ɫʯ����Һ��
| ʵ����� | Ԥ������ | ������� |
| ����1��ȡ����C����a�Թܣ�������������ˮ���� | | |
| ����2��ȡ����C��ϡ��Һ��װb��c��֧�Թܣ���b�Թ� | ������ɫ���� | |
| ����3����c�Թ� | | C�������Լ�������ɫ��Ӧ�� |
��4����ȡһ������C��������ˮ�ܽ��ȫ��ת����1000mL����ƿ�ж��ݡ�ȡ����Һ 25.00mL������Ũ��Ϊ0.0500 moL��L��1����ˮ��Һ30.00mL�����á�����Ӧ��ȫ���������KI������0.1100 moL?L��1Na2S2O3����Һ�ζ����ɵ�I2����ȥNa2S2O3����Һ11.80mL����������C���ʵ����ļ������ʽΪ�� �� �����ַ�Ӧ���ӷ���ʽ��I2+2S2O32��=2I��+S4O62����
��17�֣�
��1��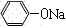 + CO2 + H2O
+ CO2 + H2O 
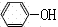 + NaHCO3 ��3�֣�
+ NaHCO3 ��3�֣�
��2����Һ ��3�֣�
��3������8�֣����������֣�ʵ����� Ԥ������ ������� ����1��ȡ����C����a�Թܣ�������������ˮ����
���ְ�ɫ���ǣ�1�֣�
���ӳ������ܽ�Ȳ��� ��1�֣�
����2��ȡ����C��ϡ��Һ��װb��c��֧�Թܣ���b�Թ����������1�֣�������ˮ��1�֣�����
������ɫ����
�������巢��ȡ����Ӧ���������屽�Ӱ�ɫ��������1�֣�
����3����c�Թ����뼸�Σ�1�֣�FeCl3��Һ��1�֣����� ��Һ��Ϊ��ɫ
��1�֣�C�������Լ�������ɫ��Ӧ��
��4��(0.0500mol/L��30.00��10��3L ��0.1100 mol/L��11.80��10��3��1/2L) ��1/ 3��1000/25.00
��3�֣����������֣�
���������������1�����ӡ����ᶼ�������ԣ������ԣ�����>̼��>����>̼��������ӣ�������з�ӦΪC6H5OH+NaOH��C6H5ONa+H2O��CH3COOH+NaOH��CH3COONa+H2O��������з�ӦΪC6H5ONa+H2O+CO2��C6H5OH+NaHCO3����2�����ڱ��ӵķе�Ϊ182�棬�����Ϊ���õ����ӣ�����ˮ�����Ƶ�Ŀ���dz�ȥˮ�֣��������μ���ᾧˮ����ķе�ߡ��ѻӷ�����BΪ���б��ӵ��л��࣬��DΪ���������ơ�̼�����Ƶ����ʵ�ˮ�࣬�ɴ��ƶϲ�����з����л���IJ����Ƿ�Һ����3�����ڱ��ӵ��۵�Ϊ43�棬�������±�������ɫ������壬���ڱ����������Ҵ����л��ܼ�����������ˮ�е��ܽ�Ƚ�С��ȡ����C����a�Թܣ�������������ˮ�������ְ�ɫ���ǣ�֤�������±����ܽ�Ȳ������ڱ���ϡ��Һ��Ũ��ˮ��Ӧ������ɫ�����屽�ӳ��������Ȼ�����Һ����ɫ������2��b�Թ���Ӧ�μ������α�����ˮ����������ɫ������˵���������巢��ȡ����Ӧ���������屽�Ӱ�ɫ����������3����c�Թܵ��뼸���Ȼ�����Һ������Һ��Ϊ��ɫ��˵���������Ȼ���������ɫ��Ӧ����4������n=c?V��n(Na2S2O3)=0.1100��11.80��10��3mol������Na2S2O3�ǿ������Σ���n(S2O32��)=n(Na2S2O3)= 0.1100��11.80��10��3mol������I2+2S2O32��=2I��+S4O62���еⵥ���������������ӵ�ϵ��֮�ȵ������ʵ���֮�ȣ���n(I2)= n(S2O32��)/2=0.1100��11.80��10��3��1/2mol������Br2+2KI=2KBr+I2��������ϵ��֮�ȵ������ʵ���֮�ȣ������û���Ӧ��n(Br2)=n(I2)= 0.1100��11.80��10��3��1/2mol������n=c?V����ˮ��n(Br2)=0.0500��30.00��10��3mol��˵���뱽�ӷ�Ӧ��n(Br2)=(0.0500��30.00��10��3��0.1100��11.80��10��3��1/2)mol������1mol������3molBr2����ȡ����Ӧ����25.00mL��Һ��n(����) =(0.0500��30.00��10��3��0.1100��11.80��10��3��1/2)��1/3mol�����ƳɵĴ�����Һ��ζ�ʱ�Ĵ�����Һ�����֮��Ϊ1000��25.00����1000mL��Һ��n(����) =(0.0500��30.00��10��3��0.1100��11.80��10��3��1/2)��1/3��1000/25.00mol�����������б��ӵ����ʵ���Ϊ(0.0500��30.00��10��3��0.1100��11.80��10��3��1/2)��1/3��1000/25.00mol��
���㣺����̽��ʵ�鷽������Ƽ���ѧ���㣬�漰���ֽⷴӦ����ѧ����ʽ�������ķ����ᴿ�����鱽����Ҫ����ʵ�鷽������ơ����ʵ���Ũ�ȡ���Һ��������ʵ��������ʵ����ڻ�ѧ�����ӷ���ʽ�����е�Ӧ�õȡ�

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�����Լռ����������71%����ˮ�л�ѧ��Դ�����þ��зdz�������ǰ����
��1��Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�
�� �Լ�����ѡ��ʯ�����ұ��������������Ŀ���� .
�� þ�������� ������ţ�������ȴ��õ�þ��
| A��N2 | B��CO2 | C��Ar | D�������� |

ij����С����ʵ����ģ�����������������װ�ý���ʵ�飨��������Ʒ���ѱ��������г�װ������ȥ����

��Aװ����ͨ��a����ʱ����Ӧ�����ӷ���ʽ��ʾΪ�� .
��Aװ����ͨ��a����һ��ʱ���
ֹͣͨ�룬��ͨ�ȿ�����ͨ���ȿ�����Ŀ���ǣ� .
�ݷ�Ӧ�����У�Bװ���в�����������Ҫ������Ϊ .
��Cװ�õ������� ��
��ҵ��ұ����ͭ��mCu2O��nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���塣
���������գ�
��1������A�еĴ�����Ⱦ���ѡ�������Լ��е� ������ţ����ա�
a. ŨH2SO4 b. ŨHNO3 c. NaOH��Һ d. ��ˮ
��2����ϡH2SO4 ��������B��ȡ����������Һ���μ� �����������ƣ���Һ��ʺ�ɫ��˵����Һ�д���Fe3+��������Һ�л�����Fe2+�ķ����� ��ע���Լ�������
|

��3����ͭұ����ͭ�Ļ�ѧ����ʽ�� ��
��4��װ����þ���������� ����ͭ�����ۻ������渲��������ɫ����a��
a�� (������)��ɳ���ܷ�ˮ�� (��ܡ����ܡ�)��
��5���õζ����ⶨCuSO4��5H2O�ĺ�����ȡa g�������100 mL��Һ��ȡ20.00mL��c mol /L �ζ���(H2Y2�C���ζ����������ʷ�Ӧ)�ζ����յ㣬���ĵζ���bmL���ζ���Ӧ��Cu2+ + H2Y2�C��CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ��
��6�����в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���_____________��
a���ζ��ٽ��յ�ʱ����ϴƿ�е�����ˮϴ�µζ��ܼ���ڵİ�α�Һ����ƿ��
b���ζ���������ˮϴ�Ӻ�ֱ��ע�����Һ��ȡ20.00mL���еζ�
c���ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ
ʵ���������᳧��������Ҫ�ɷ�ΪFe2O3������FeS��SiO2�ȣ��Ʊ���������ʽ�������ľۺ�����̷�(FeSO4?7H2O)���йصĹ����������£�
��1�������̢��в���������ͨ��������Һ�У���Һ������ɫ����___________�����ţ���
| A��Ʒ����Һ | B����ɫʯ����Һ |
| C������KMnO4��Һ | D����ˮ |
��3���ڢ��У�������������___________________________��
��4���ڢ��У�����Ũ����Ҫ�Ĺ������������ƾ����⣬����_______________________��
��5���ڢ��У�����ҺZ���Ƶ�70��80���Ŀ����________________________________��
��6��Ϊ����������Ʒ����Ԫ�ص�������������������ʵ�飨���������в�����Ԫ�غ���Ԫ�أ�����ȡ2.700g��Ʒ������Ʒ��������������μӹ�����BaCl2�����ˡ�ϴ�ӡ�����������������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������[Mr (BaSO4) =233��Mr (Fe) =56]����д��������̣��������4λ��Ч���֣���
S2C12��һ�ֽ��ɫ�ӷ���Һ�壬������������ij��ѧ��ȤС�������ʵ���Ʊ�������S2C12����������֪S2Cl2��ˮ�������绯��Ӧ��һ������Ԫ�ػ��ϼ����ߣ���һ���ֻ��ϼ۽��ͣ����������������ʺ��������Cl2��Ӧ��������S2C12����Ӧ�Ļ�ѧ����ʽΪ��2S+Cl2 S2Cl2��
S2Cl2��
��Ӧ�漰�ļ������ʵ��۷е����£�
| ���� | S | S2Cl2 |
| �е�/�� | 445 | 138 |
| �۵�/�� | 113 | -76 |
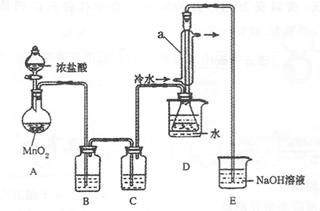
��С����Ƶ��Ʊ�װ������ͼ���г���������ȥ
�ش��������⣺
��1�����Ӻ�ʵ��װ�ú�ĵ�һ��ʵ�������______��
��2��ʵ������Ҫ���ȵ�������______������д��ĸ��
��3��װ��B��C�е��Լ��ֱ���______��
��4��װ��D������a��������______��
��5����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ�����____________��
��6����ʵ�������ȱ��Cװ�ã����ֲ�Ʒ���Dz��壬���û�ѧ����ʽ��ʾ��ԭ��____________
��7��ʵ����ϣ�С���е�һλͬѧ��ʣ��Ũ���ᵹ��E�ձ��У������л���ɫ�ݼ�����������������ӷ���ʽ��ʾ�����������ԭ��____________��
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�
��1��ָ����ȡ��Ĺ������й�ʵ��������� �� ��
��2��д�����̢����йط�Ӧ�����ӷ���ʽ
��3����ȡ��Ĺ����У��ɹ�ѡ����л��Լ���
| A������ | B���� | C�����Ȼ�̼ | D���ƾ� |
| �л��Լ� | �ƾ� | ���Ȼ�̼ | �� | ���� |
| ���� | �� | �� | �� | �� |
| ˮ���� | ��ˮ������Ȼ��� | ��ˮ�������� | ��ˮ�������� | ��ˮ������Ȼ��� |
��������þ�Ĺ����д������·�Ӧ��
������ͼװ�ö����ղ�����������зֲ����ջ��ռ���
|
|

A ��NaOH��Һ CO B��Na2CO3��Һ SO2
C�� ϡ���� S D��KMnO4��Һ CO
������ͼ��ʾװ����ȡ���������ռ���������(����)
| A��п��ϡ���ᷴӦ������ |
| B��Ũ��ˮ���������ƹ��巴Ӧ�ư��� |
| C���������ƹ��������ᷴӦ�ƶ������� |
| D��ͭ��ϡ���ᷴӦ��һ������ |
 CH3CH2CH2CHO
CH3CH2CH2CHO CH3CH2CH2CH2OH;CO���Ʊ�ԭ��:HCOOH
CH3CH2CH2CH2OH;CO���Ʊ�ԭ��:HCOOH CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
CO��+H2O,����Ƴ�ԭ�������Ʊ�װ��(��ͼ)��
