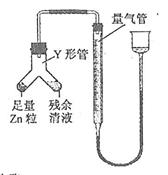
��Ŀ����
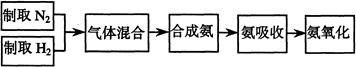
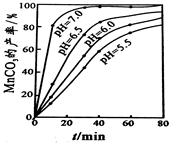
��15�֣�����ʯ���ʵĸ���Ʒ����������(Na2SiF6)���Ʊ���ʯ(Na3AlF6)������ʯ�ǵ���������ۼ�,�ɽ������������۵㡣��ͼ�ǹ�ҵ����������ͼ��
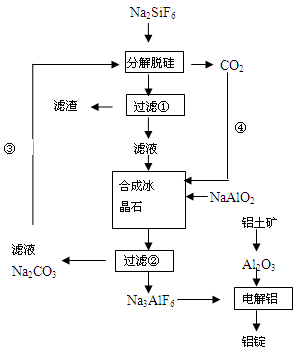
��1����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ����������__________�����ڣ���һ����Ӧ�ķ���ʽ______________________________________________________________
��2�����Ʊ������������ι��˲��������˲����ٵ���Һ��________��Һ��������________ ��
��3���ֽ��ѹ�ͺϳɱ���ʯ��ѧ��Ӧ����ʽ�ֱ�Ϊ��_________________��____________________��
��4�����չ����Тۺܵ͢�Ŀ����_____________________��̼���ƺͶ�����̼�Ƿ��� ��
��5�����Al2O3��Alʱ��I=200kA��һ����Al 1��430 t�����Ч���Ƕ��٣�

��1����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ����������__________�����ڣ���һ����Ӧ�ķ���ʽ______________________________________________________________
��2�����Ʊ������������ι��˲��������˲����ٵ���Һ��________��Һ��������________ ��
��3���ֽ��ѹ�ͺϳɱ���ʯ��ѧ��Ӧ����ʽ�ֱ�Ϊ��_________________��____________________��
��4�����չ����Тۺܵ͢�Ŀ����_____________________��̼���ƺͶ�����̼�Ƿ��� ��
��5�����Al2O3��Alʱ��I=200kA��һ����Al 1��430 t�����Ч���Ƕ��٣�
��1���� Al2O3+2NaOH=2NaAlO2+H2O
��2��NaF SiO2
��3��2Na2CO3+Na2SiF6=SiO2��+2CO2��+ 6NaF 6NaF +NaAlO2+2CO2=Na3AlF6��+2Na2CO3
��4��Na2CO3��CO2ѭ��ʹ�� �������ã�������ľ��貹��
��5��88��7%
��2��NaF SiO2
��3��2Na2CO3+Na2SiF6=SiO2��+2CO2��+ 6NaF 6NaF +NaAlO2+2CO2=Na3AlF6��+2Na2CO3
��4��Na2CO3��CO2ѭ��ʹ�� �������ã�������ľ��貹��
��5��88��7%
�����������1����ҵ�ϴ��������Ʊ��ϸߴ���Al2O3����Ҫ�������̣����ȼ�������NaOH��Һ��ʹAlת��ΪAlO2-,�������Һ�м���������������ᣬ��ʱAl��ΪAl3+��Ȼ����������İ�ˮ�õ�Al(OH)3������������˳�����ϴ�Ӹɾ�����ɡ��������Al(OH)3�õ�������Al2O3�������Ҫ�������ڡ���һ����Ӧ�ķ���ʽAl2O3+2NaOH=2NaAlO2+H2O����2�����Ʊ������������ι��˲�����������ͼ��֪���ڹ��˲����ٵ���Һ��NaF��������SiO2����3���ֽ��ѹ軯ѧ��Ӧ����ʽ��2Na2CO3+Na2SiF6=SiO2��+2CO2��+ 6NaF���ϳɱ���ʯ��ѧ��Ӧ����ʽ�ֱ�Ϊ��6NaF +NaAlO2+2CO2=Na3AlF6��+2Na2CO3 ����4�����չ����Тۺܵ͢�Ŀ����Na2CO3��CO2ѭ��ʹ�ã�������ʵ������ʡ����ܡ��������ɵ������ķ���ʽ��֪��������̼���ƺͶ�����̼ǡ����ȫ��Ӧ������ʵ��Ӧ��ʹ�������ʵ������ʲ�����100%�����Ծ��貹�䡣��5��һ����Al 1��430 t��n(e-)=1��430��106g��27g/mol��3=1��6��105mol������Q=1��6��105��6��02��1023��1��6��10-19=1��54��1010,���ĵĵ���n(e-)=2��105��3600��24=1��73��1010�����Ե��Ч���ǣ�1��54��1010��1��73��1010����100%=88��7%��

��ϰ��ϵ�д�
�����Ŀ
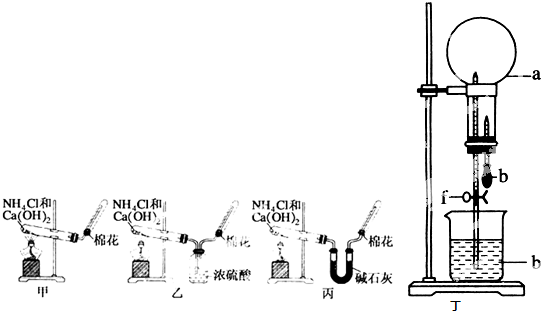


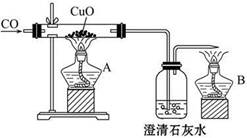

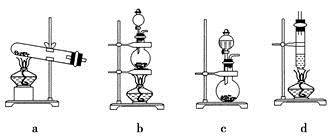
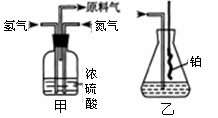
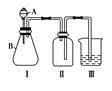 (4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
(4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺