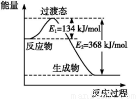
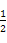��Ŀ����
����˵����ȷ���ǣ� ��
A.þ�뼫ϡ���ᷴӦ��������淋����ӷ���ʽΪ:4Mg+6H++N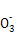 =4Mg2++N
=4Mg2++N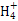 +3H2O
+3H2O
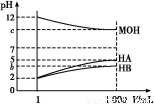
B.������,0.1 mol/LһԪ��HA��Һ��c(OH-)/c(H+)=1��10-8,�����Һ��pH=3
C.��ͬ������,Ũ�Ⱦ�Ϊ0.01 mol/L��NH4Cl��Һ��NaCl��Һ��,ǰ�ߵ���������Ũ�ȴ��ں��ߵ���������Ũ��
D.���ʵ���Ũ����ȵĴ��������������Һ�������Ϻ����Һ��:c(Na+)+c(H+)=c(CH3COO-)+c(OH-)+c(CH3COOH)
B
��������A���ɡ�ԭ�Ӳ��غ�,ӦΪ4Mg+10H++N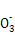 =4Mg2++N
=4Mg2++N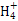 +3H2O,����;c(OH-)/c(H+)=1��10-8��c(OH-)��c(H+)=1��10-14����,�ɵ�c(H+)=1��10-3 mol��L-1,��pH=3,B��;������Һ������,NH4Cl��Һ�д���c(N
+3H2O,����;c(OH-)/c(H+)=1��10-8��c(OH-)��c(H+)=1��10-14����,�ɵ�c(H+)=1��10-3 mol��L-1,��pH=3,B��;������Һ������,NH4Cl��Һ�д���c(N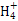 )+c(H+)=c(Cl-)+c(OH-),NaCl��Һ�д���c(Na+)+c(H+)=c(Cl-)+c(OH-),����NH4Cl��Һ�����Զ�NaCl��Һ������,��ǰ�ߵ�c(OH-)С�ں���,��c(Cl-)��Ϊ0.01 mol/L,��c(N
)+c(H+)=c(Cl-)+c(OH-),NaCl��Һ�д���c(Na+)+c(H+)=c(Cl-)+c(OH-),����NH4Cl��Һ�����Զ�NaCl��Һ������,��ǰ�ߵ�c(OH-)С�ں���,��c(Cl-)��Ϊ0.01 mol/L,��c(N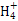 )+c(H+)��c(Na+)+c(H+),C��;������Һ������,CH3COONa��Һ�д���c(Na+)+c(H+)=c(CH3COO-)+c(OH-),D����
)+c(H+)��c(Na+)+c(H+),C��;������Һ������,CH3COONa��Һ�д���c(Na+)+c(H+)=c(CH3COO-)+c(OH-),D����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
| ���ʵ����ʵ��� Ũ��/mol��L��1 | ��Һ���ܶ�/g��cm��3 |
���� | c1 | ��1 |
��ˮ | c2 | ��2 |

��1�������������������Ϊ________(��д��λ���ú�c1����1�Ĵ���ʽ��ʾ)��
��2�����ʵ���Ũ��Ϊc1 mol��L��1��������ˮ��������(��Ϻ���Һ������仯���Բ���)��������Һ�����ʵ���Ũ��Ϊ________ mol��L��1��
��3�������ʵ���Ũ�ȷֱ�Ϊc2 mol��L��1��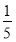 c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(��������������С������������������ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________
c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(��������������С������������������ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________ 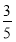 c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��
c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��