��Ŀ����
����Ŀ����̼����(Na2CO4)��һ�ֺܺõĹ�����������ϡ���ᷴӦ�Ļ�ѧ����ʽΪ:2Na2CO4+4HCl=4NaCl+2CO2��+O2��+2H2O�����۹�̼����һ�㶼����̼���ƣ�Ϊ�ⶨij��̼������Ʒ(ֻ��Na2CO4��Na2CO3)�Ĵ��ȣ�ij��ѧ��ȤС������������ַ���ʵʩ:
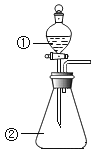
����һ:[��Ʒ] ![]() [��Ʒm1g]
[��Ʒm1g] ![]() [��Һ]
[��Һ] ![]() [����]
[����] ![]() [����m2g]
[����m2g]
��1�������ٵ�����Ϊ___________��
��2�����������У�ʹ�õ�����������________(��������)��
��3������������۵IJ�������_________________��
������:����ͼ��װ��ʵ��װ�ã�QΪһ��������������ȡ������Ʒ�����У���Һ©����������ϡ�����������������ַ�Ӧ��
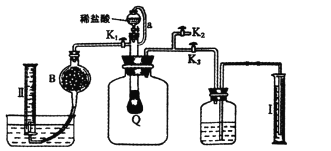
��4��Ϊ�ⶨ��Ӧ������������������ϡ����ǰ����ر�____����_____(����K1������K2������K3��)��
��5����������Ӧֹͣ��ʹK1��K3���ڹر�״̬��K2���ڴ�״̬���ٻ�����K1��B��װ�Ĺ����Լ���______��Ϊ��Ҫ������K1?__________________��
��6��ʵ�����ʱ����ͲI����xmLˮ������II���ռ�����ymL���壬����Ʒ�й�̼���Ƶ�����������_____________��
���𰸡� ���� �ڢ� �����ᾧ��������ϴ�������� K1��K2 K3 ��ʯ�� �����ɵ�CO2�ܳ�ֱ�B�м�ʯ�����գ�ʹ��Ͳ���ռ����ϴ�����O2����Сʵ����� ![]()
����������������������е�ʵ�鲽���֪������һ�ⶨ��̼������Ʒ(ֻ��Na2CO4��Na2CO3)�Ĵ��ȵ�ԭ���ǣ�����һ����������Ʒ������������ϡ�������䷴Ӧ��������Һ�����ᾧ��������ϴ��������õ��Ȼ��ƾ��壬���������Ȼ��Ƶ�������������Ʒ������m1g���Ȼ��Ƶ�����m2g���������̼���ƵĴ��ȡ���������ԭ���ǣ�ȡһ��������Ʒ��������������ϡ���ᷴӦ��ͨ����ͲI�����������������������徭B�еļ�ʯ�����պ���ͨ����ͲII��������������������������������ȿ��������Ʒ�й�̼���ƵĴ��ȡ�
��1�������ٵ�����Ϊ������
��2�����������У������ܽ⡢���˶�Ҫʹ�õ�����������ѡ�ڢ���
��3�������۵IJ��������ǣ������ᾧ��������ϴ����������
��4��Ϊ�ⶨ��Ӧ������������������ϡ����ǰ����ر�K1��K2����K3������������ѹ��ƿ�е�ˮ������ͲI�У����ɲⶨ��Ӧ����������������
��5����������Ӧֹͣ��ʹK1��K3���ڹر�״̬��K2���ڴ�״̬���ٻ�����K1��B��װ�Ĺ����Լ�Ӧ�����ն�����̼���壬��õ�ѡ���Ǽ�ʯ�ң�������K1��Ŀ���ǣ������ɵ�CO2�ܳ�ֱ�B�м�ʯ�����գ�ʹ��Ͳ���ռ����ϴ�����O2����Сʵ�������
��6��ʵ�����ʱ����ͲI����xmLˮ������II���ռ�����ymL���壬��O2�����ΪymL��CO2�����Ϊ(x-y)mL��n(O2):n(CO2)=![]() ����2Na2CO4+4HCl=4NaCl+2CO2��+O2��+2H2O��Na2CO3+2HCl=2NaCl+CO2��+H2O��֪����Ʒ��
����2Na2CO4+4HCl=4NaCl+2CO2��+O2��+2H2O��Na2CO3+2HCl=2NaCl+CO2��+H2O��֪����Ʒ��![]() ������
������![]() ����̼���Ƶ�����������
����̼���Ƶ�����������![]() ��
��