��Ŀ����
����Ŀ������������Ԫ��A��B��C��D�����ǵ�ԭ��������������A��Dͬ����������������Ϊ������AΪԪ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ�ӵ������������Ǵ�����������2����CΪ�ؿ��к�������Ԫ�ء���ش��������⣺
��1��CԪ�ص����ӽṹʾ��ͼ________��CԪ���γɵij���Һ̬������Ľṹʽ________��
��2��DԪ����Ԫ�����ڱ��е�λ��__________��д��D��C������ȼ���γɵĻ�����ĵ���ʽ__________��
��3��B��C�ֱ��γɵļ���̬�⻯���н��ȶ�����______������B�γɵļ���̬�⻯��Ŀռ�ṹ _______������B��C��Ӧ���ɵ�XY2�ͻ�����ĵ���ʽ______________��
��4������B�������������D������������Ӧ��ˮ�������Һ��Ӧ�����ӷ���ʽ__________________________________________________________________��
��5������ʽΪB6A14�����ʺ��ж���ͬ���칹�壬����һ��ͬ���칹���һ�ȴ���ֻ�����֣�д���������ʵĽṹ��ʽ______________��
���𰸡�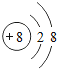 H-O-H �������ڵڢ�A�壨���3���ڵڢ�A����
H-O-H �������ڵڢ�A�壨���3���ڵڢ�A���� ![]() H2O ��������
H2O �������� ![]() CO2+2OH��=CO32-+H2O CH3CH��CH3��CH��CH3��2
CO2+2OH��=CO32-+H2O CH3CH��CH3��CH��CH3��2
��������
AΪԪ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ����CΪ�ؿ��к�������Ԫ�أ���CΪOԪ�أ�����B�Ľṹ��������������Ӧ�ֱ�Ϊ2��4��BӦΪCԪ�أ�����ԭ������������A��Dͬ�����ȷ��D��NaԪ�����ݴ˴��⡣
��1��CΪ��Ԫ�أ�������Ϊ8��������С�ں���������������ӽṹʾ��ͼΪ��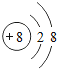 ����Ԫ���γɵij���Һ̬��������ˮ�����ĽṹʽΪH-O-H���ʴ�Ϊ��
����Ԫ���γɵij���Һ̬��������ˮ�����ĽṹʽΪH-O-H���ʴ�Ϊ��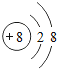 ��H-O-H��
��H-O-H��
��2��DΪ��Ԫ�أ���Ԫ��λ�����ڱ��ĵ������ڵڢ�A�壬����������ȼ�����ɹ������ƣ����������мȺ����Ӽ��ֺ����ۼ�������ʽΪ��![]() ���ʴ�Ϊ���������ڵڢ�A�壨���3���ڵڢ�A������
���ʴ�Ϊ���������ڵڢ�A�壨���3���ڵڢ�A������![]() ��
��
��3��B��C����Ԫ�����γɵ���̬�⻯��ֱ�ΪCH4��H2O���ǽ�����Խǿ����̬�⻯��Խ�ȶ����ǽ�����O��C���ȶ���CH4��H2O������CH4��H2O���ȶ�����H2O��B�γɵļ���̬�⻯����CH4��CH4�Ŀռ乹�����������壻B��C��Ӧ���ɵ�XY2�ͻ�����ΪCO2��CO2���ɷǽ����γɵĹ��ۼ���CO2�ĵ���ʽΪ��![]() ���ʴ�Ϊ��H2O������������
���ʴ�Ϊ��H2O������������![]() ��
��
��4��B�������������CO2��D������������Ӧ��ˮ������NaOH����Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O�����ӷ���ʽΪ��CO2+2OH��=CO32-+H2O���ʴ�Ϊ��CO2+2OH��=CO32-+H2O��
��5������ʽΪB6A14������ΪC6H14��ͬ���칹����5����CH3CH2CH2CH2CH2CH3����CH3��2CHCH2CH2CH3��CH3CH2CH��CH3��CH2CH3����CH3��2CHCH��CH3��2����CH3��3CCH2CH3��
һ�ȴ���������
CH3��CH2��4CH3�����ֲ�ͬ������ԭ�ӣ�����һ�ȴ�����3�֣�
CH3CH��CH3����CH2��2CH3�����ֲ�ͬ������ԭ�ӣ�����һ�ȴ�����5�֣�
CH3CH2CH��CH3��CH2CH3�����ֲ�ͬ������ԭ�ӣ�����һ�ȴ�����4�֣�
CH3C��CH3��2CH2CH3�����ֲ�ͬ������ԭ�ӣ�����һ�ȴ�����3�֣�
CH3CH��CH3��CH��CH3��2�����ֲ�ͬ������ԭ�ӣ�����һ�ȴ�����2�֣�
�ʴ�Ϊ��CH3CH��CH3��CH��CH3��2��

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�