��Ŀ����
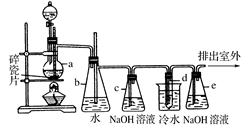
ij��ѧС���������������������װ�ã���ͼ�����Ի�����Ϊ��Ҫԭ���Ʊ�����ϩ��
(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���l mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ���(�g����f��)���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________________________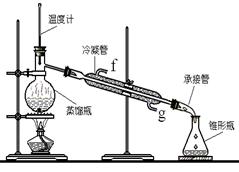
��1���ٷ�ֹ���ҷ��� ���� ��ʹ����ϩҺ�������ٻӷ�
��2�����ϲ� C ��g ��83 ��
�Ʊ���Ʒʱ���������Ʒһ������
���������������1����A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�������������ʹ����ϩҺ����
�ڻ���ϩ���۷е�ͣ������Թ�C���ڱ�ˮԡ�е�Ŀ����ʹ����ϩҺ�������ٻӷ���
��2���ٻ���ϩ���ܶȱ�ˮС�����Լ��뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ㣻��������д�������ϴ�ӣ��ױ����������������������ʣ�������ϡH2SO4������ѡ��̼������Һ���������е��������ʷ�Ӧ����ѡC��
������ʱ��ȴˮ���¶˽����϶˳���������ȴˮ��g�ڽ�������ϩ�ķе���83�棬���Կ��Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ�����Ʊ���Ʒʱ���������Ʒһ��������ʹ���ʽ��͡�
���㣺�����ʵ��װ�����õ��жϣ���ʵ�����ķ���

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д���ȥ���������������������ʣ���д��ѡ�õ��Լ��ͷ��뷽��
| | ����� (������Ϊ��������) | �Լ� �������� | ���뷽�� |
| A | �������ӣ� | | |
| B | ��ϩ��SO2�� | | |
| C | �������������ᣩ | | |
| D | �Ҵ���ˮ�� | | |
��15�֣�CoCl2��6H2O��һ������Ӫ��ǿ������һ������ˮ�����Ҫ�ɷ�ΪCO2O3��Co
��OH��3����������Fe2O3��Al2O3��MnO�ȣ���ȡCoCl2��6H2O�Ĺ����������£�
��֪���ٽ���Һ���е���������Ҫ��H+��CO2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±�������������Ũ��Ϊ��0��01 mo1��L-l��
��CoCl2��6H2O�۵�Ϊ86�棬������110��120��ʱ��ʧȥ�ᾧˮ������ˮ�Ȼ��ܡ�
��1��д������������Co2O3������Ӧ�����ӷ���ʽ ��
��2��д��NaC1O3������Ӧ����Ҫ���ӷ���ʽ ������������Һ���мӹ���NaClO3ʱ�����ܻ������ж����壬д�����ɸ��ж���������ӷ���ʽ ��
��3������Na2CO3��pH��a'�����������õ��ij����ɷ�Ϊ____ ��
��4����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ������Һ���м�����ȡ����Ŀ���� ����ʹ�õ����pH��Χ��____ ��
| A��2��0��2��5 | B��3��0��3��5 | C��4��0��4��5 | D��5��0��5��5 |
��6��Ϊ�ⶨ�ֲ�Ʒ��CoCl2��6H2O��������ȡһ�������Ĵֲ�Ʒ����ˮ����������AgNO3��Һ�����ˡ�ϴ�ӣ���������ɺ����������ͨ�����㷢�ֲִ�Ʒ��CoCl2��6H2O��������������100%����ԭ������� ������һ�����ɣ�
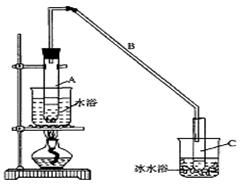
ʵ��������������ˮ���������Ҵ��Ʊ�1��2�����������װ������ͼ��ʾ��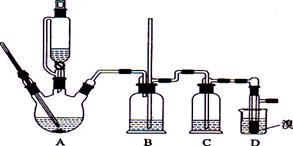
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g �� cm-3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | -l30 | 9 | -1l6 |

�ش��������⣺
��1����ƿD�з�������Ҫ�ķ�Ӧ����ʽ ��
��2����ȫƿB���Է�����,�����Լ��ʵ�����ʱ�Թ�D�Ƿ�����������д����������ʱƿB�е����� ��
��3����װ��C��Ӧ���� (����ĸ) ����Ŀ����_______________��
a��ˮ b��Ũ���� c������������Һ
��4����������������δ��Ӧ��Br2������� ϴ�ӳ�ȥ��������ĸ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��ȡ�������Ҫ���������� ��
��5�������������������������ѣ����� �ķ�����ȥ��
��6����Ӧ������Ӧ����ˮ��ȴװ��D�����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���� ��
��7���жϸ��Ƹ���Ӧ�Ѿ�������������� ��
�����״���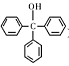 ����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��
����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��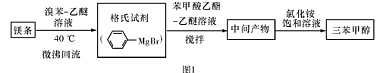
��֪����I�������Լ�����ˮ�⣺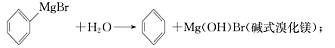
����������ʵ������������£�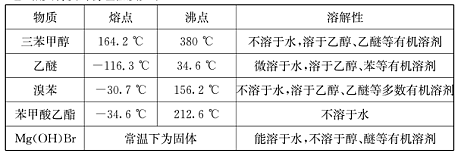
���������״�����Է���������260�����������л���һ�㶼�й̶��۵㡣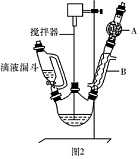
��ش��������⣺
��1��ͼ2�в�������B�����ƣ� ��װ����ˮCaCl2������A�������� ��
��2��ͼ2�еμ�Һ��δ����ͨ��Һ©�����õ�Һ©���������� ����ȡ�����Լ�ʱҪ�����У����Բ��� ���ȷ�ʽ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д���¿հף�
���У��ٲ���Ϊ�� ��ϴ��Һ���ѡ�� ��������ѡ����ѡ��
| A��ˮ | B������ | C���Ҵ� | D���� |
��4�����Ȳⶨ����ȡ2��60 g��Ʒ�����������Һ���������������ƣ��������Ʋ���Ӧ������ַ�Ӧ������ɵ������ڱ�״���µ����Ϊ100.80mL�����Ʒ�������״�����������Ϊ ��
�����л�����ϩ������ȥ��ϩ�õ������ļ��飬�������ͨ��ʢ��������Щ�Լ���ϴ��ƿ�� ��
| A������ʯ��ˮ��ŨH2SO4 | B������KMnO4��ŨH2SO4 |
| C����ˮ��ŨH2SO4 | D��ŨH2SO4����ˮ |

 3NH3 + 8AlO2��
3NH3 + 8AlO2��