��Ŀ����
7��ij��ѧ��ȤС����Ҫ����6mol/L��H2SO4���������ֲ�ͬŨ�ȵ������240mL 1mol/L ��150mL 4mol/L ��������18mol/L������ƿ�����ֹ��250mL��500mL��1000mL�������ù����У���ȤС�齫�٢��������������ˣ���ش�����������1����ȤС���Ƿ�����18mol/L�������أ�
��2����ȤС�������ƹ�������ѡ�õ�����ƿ�Ĺ����1000mL��ͨ������˵����
���� ��1���٢���Һ��Ũ�Ⱦ�С��6mol/L������Ҫ�õ�18mol/L�����
��2������Ҫ��18 mol/L��Ũ����VmL�����ʵ����ʵ���Ϊ0.24L��1mol/L+0.15L��4mol/L+0.00VL��18mol/L=0.64mol+0.018Vmol��
������Һ��Ũ�ȣ�$\frac{0.64mol+0.018Vmol}{0.39L+0.00VL}$=6mol/L��V=0.142L=142mL����Һ�����290mL+142mL=536mL����Ҫ1000mL������ƿ��
��� �⣺��1���٢���Һ�����Ϊ390mL�����ǵ�Ũ�Ⱦ�С��6mol/L������Ҫ�õ�18mol/L�����ᣬ������18mol/L�����
��2������Ҫ��18 mol/L��Ũ����VmL�����ʵ����ʵ���Ϊ0.24L��1mol/L+0.15L��4mol/L=0.84mol��
������Һ��Ũ�ȣ�$\frac{0.64mol+0.018Vmol}{0.39L+0.00VL}$=6mol/L��V=0.142L=142mL����Һ�����290mL+142mL=536mL����Ҫ1000mL������ƿ��
�ʴ�Ϊ��1000 mL��
���� ���⿼��������һ�����ʵ���Ũ�ȵ����Ƽ��㣬������ѵ���������ƿ����ѡ���ѶȽϴ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
17����0.2mol������̬����ɵĻ��������ȫȼ�պ�õ�6.72��������̼����״���£���7.2��ˮ������˵����ȷ���ǣ�������
| A�� | һ������ϩ | B�� | һ��û������ | C�� | һ��û�м��� | D�� | ��ȷ�� |
15�����л����ﲻ�����ӻ�������ǣ�������
| A�� | SiO2 | B�� | KHCO3 | C�� | NH4Br | D�� | NaOH |
12��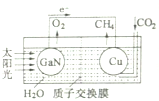 ��ѧ���õ����ز�����ͭ��װ����ͼ���˹����ϵͳ��ʵ������CO2��H2OΪԭ�Ϻϳ�CH4�������й�˵������ȷ���ǣ�������
��ѧ���õ����ز�����ͭ��װ����ͼ���˹����ϵͳ��ʵ������CO2��H2OΪԭ�Ϻϳ�CH4�������й�˵������ȷ���ǣ�������
 ��ѧ���õ����ز�����ͭ��װ����ͼ���˹����ϵͳ��ʵ������CO2��H2OΪԭ�Ϻϳ�CH4�������й�˵������ȷ���ǣ�������
��ѧ���õ����ز�����ͭ��װ����ͼ���˹����ϵͳ��ʵ������CO2��H2OΪԭ�Ϻϳ�CH4�������й�˵������ȷ���ǣ�������| A�� | ��װ���ǽ���ѧ��ת��Ϊ���� | |
| B�� | �����ص缫������ | |
| C�� | ͭ�缫����ĵ缫��ӦʽΪ��CO2+8e-+8H+=CH4+2H2O | |
| D�� | ������������װ�õĵ��Һ |
10��NA��ʾ�����ӵ�������ֵ������˵������ȷ���ǣ�������
| A�� | ���³�ѹ�£�21g������27g����������ԭ������Ϊ3NA | |
| B�� | 34gH2O2�к���������ΪNA | |
| C�� | ����FeCl3��Һ�����ˮ�У�����1molFeCl3ˮ�⣬������NA���������� | |
| D�� | 80mL10mol/LŨ����������MnO2���ȷ�Ӧ��ת�Ƶ�����Ϊ0.4NA |
11�������Ѷ����������ж��ԣ����뼡�����������һ������С����������֮�ƣ������й�${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti��˵���в���ȷ���ǣ�������
| A�� | ${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti����ͬ��Ԫ�� | |
| B�� | ${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$Ti����������ͬ | |
| C�� | ${\;}_{22}^{48}$Ti�����ԭ������ԼΪ48 | |
| D�� | ${\;}_{22}^{48}$Ti��${\;}_{22}^{50}$TiΪ��ͬ�ĺ��أ���Ϊͬλ�� |
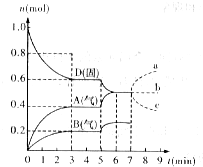 ���ݻ�Ϊ2.0L���ܱ������ڣ�����D��T��ʱ������Ӧ���䷴Ӧ�������������ʵ�����ʱ��t�ı仯��ϵ��ͼ����ͼ�ش��������⣺
���ݻ�Ϊ2.0L���ܱ������ڣ�����D��T��ʱ������Ӧ���䷴Ӧ�������������ʵ�����ʱ��t�ı仯��ϵ��ͼ����ͼ�ش��������⣺ ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��
ij��ȤС����С����ý������ᷴӦ����ʵ�飬��5.4g����ƬͶ��500mL 0.5mol•L-1��������Һ�У���ͼΪ��Ӧ�������������뷴Ӧʱ��Ĺ�ϵͼ��