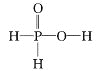
��Ŀ����
����Ŀ����Ҫ��������м���
��1��1mol CO2������Ϊ_________��
��2����״���£�11.2L NH3���еķ�����Ϊ_________ ��
��3��0.1mol/L��BaCl2��Һ�У�Cl-�����ʵ���Ũ��Ϊ__________ ��
��4����״���£�ij����������Ļ�ѧʽΪRO2�� 1.28 g������������Ϊ448 mL������������Ħ������Ϊ_______��R�����ԭ������Ϊ________��
��5��18g H2O���е�ԭ����Ϊ_________��������Ϊ________ ��
���𰸡�44g 0.05NA 0.2mol/L 64g/mol 32 3NA 10NA
��������
��1��1mol CO2������Ϊm=nM��
��2����״���£�11.2L NH3����������Ϊn= ![]() =
= ![]() =0.05mol��������Ϊ0.05NA��
=0.05mol��������Ϊ0.05NA��
��3��0.1mol/L��BaCl2��Һ�У�1��BaCl2��������Cl-����Cl-�����ʵ���Ũ��Ϊ0.2mol/L��
��4����״���£�1.28g������������Ϊ448mL������������ʵ���Ϊ��![]() =0.02mol���������Ħ������Ϊ��
=0.02mol���������Ħ������Ϊ��![]() =64g/mol����R�����ԭ������Ϊx����x+16��2=64�����x=32��RΪOԪ�أ�
=64g/mol����R�����ԭ������Ϊx����x+16��2=64�����x=32��RΪOԪ�أ�
��5��18gH2O�����ʵ���Ϊ![]() =1mol��1��H2O��������3��ԭ�ӣ��ʺ���ԭ����ĿΪ1mol��3NAmol-1=3NA��ÿ��H2O���Ӻ���10�����ӣ���n�����ӣ�=1mol��10=10mol���ʺ��е�����ĿΪ10mol��NAmol-1=10NA��
=1mol��1��H2O��������3��ԭ�ӣ��ʺ���ԭ����ĿΪ1mol��3NAmol-1=3NA��ÿ��H2O���Ӻ���10�����ӣ���n�����ӣ�=1mol��10=10mol���ʺ��е�����ĿΪ10mol��NAmol-1=10NA��
��1��1mol CO2������Ϊm=nM=1mol��44g/mol=44g����ȷ����44g��
��2����״���£�11.2L NH3����������Ϊn= ![]() =
= ![]() =0.05mol��������Ϊ0.05NA��
=0.05mol��������Ϊ0.05NA��
��3��0.1mol/L��BaCl2��Һ�У�1��BaCl2��������Cl-����Cl-�����ʵ���Ũ��Ϊ0.2mol/L��
��4����״���£�1.28g������������Ϊ448mL������������ʵ���Ϊ��![]() =0.02mol���������Ħ������Ϊ��
=0.02mol���������Ħ������Ϊ��![]() =64g/mol����R�����ԭ������Ϊx����x+16��2=64�����x=32��RΪOԪ�أ��ʴ�Ϊ��64g/mol��32��
=64g/mol����R�����ԭ������Ϊx����x+16��2=64�����x=32��RΪOԪ�أ��ʴ�Ϊ��64g/mol��32��
��5��18gH2O�����ʵ���Ϊ![]() =1mol��1��H2O��������3��ԭ�ӣ��ʺ���ԭ����ĿΪ1mol��3NAmol-1=3NA��ÿ��H2O���Ӻ���10�����ӣ���n�����ӣ�=1mol��10=10mol���ʺ��е�����ĿΪ10mol��NAmol-1=10NA���ʴ�Ϊ��3NA��10NA��
=1mol��1��H2O��������3��ԭ�ӣ��ʺ���ԭ����ĿΪ1mol��3NAmol-1=3NA��ÿ��H2O���Ӻ���10�����ӣ���n�����ӣ�=1mol��10=10mol���ʺ��е�����ĿΪ10mol��NAmol-1=10NA���ʴ�Ϊ��3NA��10NA��

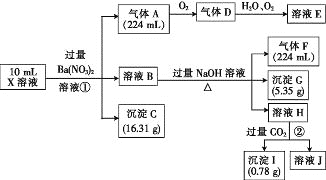
����Ŀ��������������ë֯Ʒ��������±������������ʯӲ������ɫ�����Ը���ĸ��ʯ(��4.5%Cr2O3������Fe2O3��FeO��MgO��SiO2)Ϊԭ���Ʊ��������Ĺ���������ͼ��
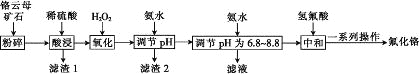
�����г�����ؽ������������������������pH��
�������� | Fe(OH)3 | Mg(OH)2 | Cr(OH)3 | Fe(OH)2 |
��ʼ������pH | 2.3 | 8.8 | 4.9 | 7.5 |
������ȫ��pH | 4.1 | 10.4 | 6.8 | 9.7 |
��ش��������⣺
(1)Fe2O3��MgO��FeO��SiO2�����ڼ������������________�֡�
(2)������ĸ��ʯ�����Ŀ����________��
(3)����1��Ҫ�ɷֵ���;��________��(дһ��)
(4)Cr2O3��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ___________��
(5)��һ�εμӰ�ˮ����pH��ΧΪ________��
(6)�ڶ��εμӰ�ˮ����pHΪ6.8~8.8��Ŀ����_______��Cr(OH)3�� Al(OH)3һ���������ԣ����ڶ��εμӵİ�ˮ��ΪNaOH��Һ�����ɵ�Cr(OH)3�Ჿ���ܽ⣬д��Cr(OH)3�ܽ�����ӷ���ʽ��________��
����Ŀ��������(CH3OCH3)��δ������������ͺ�Һ������Ϊ�ྻҺ��ȼ��ʹ�ã�
��ҵ����CO��H2Ϊԭ������CH3OCH3���¹�����Ҫ����������Ӧ��
��CO( g)+2H2(g)![]() CH3OH( g)��H1=-91kJ��mol-1
CH3OH( g)��H1=-91kJ��mol-1
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g)��H2=-24kJ��mol-1
CH3OCH3(g)+H2O(g)��H2=-24kJ��mol-1
��CO(g)+H2O(g)![]() CO2(g)+H2(g)��H3=-41kJ��mol-1
CO2(g)+H2(g)��H3=-41kJ��mol-1
�ش��������⣺
(1)�¹��յ��ܷ�ӦΪ��3CO(g)+3H2(g)![]() CH3OCH3(g)+CO2(g)���÷�Ӧ��H=______��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��________(����ĸ����)��
CH3OCH3(g)+CO2(g)���÷�Ӧ��H=______��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��________(����ĸ����)��
a�����¸�ѹ��b��������� c������CO2��Ũ��d������CO��Ũ�ȡ�e�������������
(2)��֪��Ӧ��2CH3OH(g)![]()
![]() CH3OCH3(g)��H2O(g)��ij�¶��£���1L�ܱ������м���CH3OH����Ӧ10minʱ�ﵽƽ�⣬��ʱ����ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g)��ij�¶��£���1L�ܱ������м���CH3OH����Ӧ10minʱ�ﵽƽ�⣬��ʱ����ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/(mol��L��1) | 0.01 | 0.2 | 0.2 |
��ƽ�ⳣ������ʽK=______�����¶ȵ�ƽ�ⳣ��Ϊ______��
��������CH3OH��10 min��ﵽƽ�⣬��ʱ���ڵ�ƽ����Ӧ����v(CH3OH)��________��
��ҵ�ϳ����������(��Ҫ�ɷ�ΪAs2S3)�Ƴɽ�״��ͨ��O2����������H3AsO4�͵�����д��������Ӧ�Ļ�ѧ����ʽ________________________________��