��Ŀ����
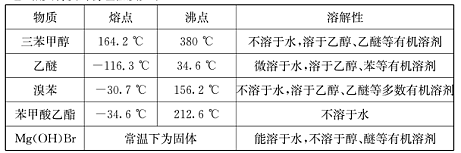
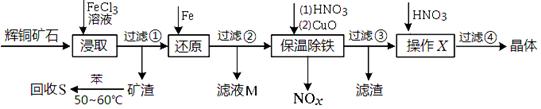
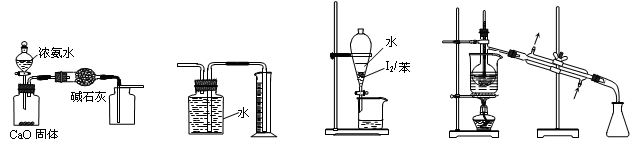
1,2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ���2��18g/cm3���е�131��4�棬�۵�9��79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1,2-�������顣���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ��(���渲������ˮ)��
(1)д���������Ʊ�1,2-���������������ѧ��Ӧ����ʽ_____________________________��
(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����________________________________________��
(3)����c��NaOH��Һ�������ǣ� ��
(4)ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬���װ�õ�������û�����⣬�Է�������ܵ�ԭ�� ��

(1)д���������Ʊ�1,2-���������������ѧ��Ӧ����ʽ_____________________________��
(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����________________________________________��
(3)����c��NaOH��Һ�������ǣ� ��
(4)ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬���װ�õ�������û�����⣬�Է�������ܵ�ԭ�� ��
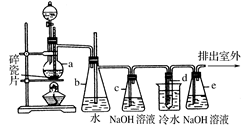
(1) CH3CH2OH  CH2=CH2����H2O�� CH2=CH2��Br2 �� CH2BrCH2Br��
CH2=CH2����H2O�� CH2=CH2��Br2 �� CH2BrCH2Br��
(2)b��ˮ����½����������е�ˮ������������������
(3)��ȥ��ϩ�д�������������(�������̼����������)��
(4)����ϩ����(��ͨ��Һ��)�ٶȹ��죬��ʵ������У���ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170��(��д�����²�����Ҳ����)
 CH2=CH2����H2O�� CH2=CH2��Br2 �� CH2BrCH2Br��
CH2=CH2����H2O�� CH2=CH2��Br2 �� CH2BrCH2Br��(2)b��ˮ����½����������е�ˮ������������������
(3)��ȥ��ϩ�д�������������(�������̼����������)��
(4)����ϩ����(��ͨ��Һ��)�ٶȹ��죬��ʵ������У���ϩ��Ũ����Ļ��Һû��Ѹ�ٴﵽ170��(��д�����²�����Ҳ����)
�����������1�����Ҵ���Ũ�����ϼ��ȷ�����ȥ��Ӧ�õ���ϩ��ˮ��Ȼ�����ϩͨ�뵽��ˮ�У������ӳɷ�Ӧ�õ�1,2-�������顣��Ӧ��������ѧ��Ӧ����ʽΪ��CH3CH2OH
 CH2=CH2����H2O�� CH2=CH2��Br2 �� CH2BrCH2Br��(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������d��������,���ڲ��������岻�ܼ�ʱ�ų���ʹװ���е�����ѹǿ����ƿbˮ����½����������е�ˮ����������������������(3)����c��NaOH��Һ�������dz�ȥ��ϩ�д�������������(�������̼����������)����ֹ��Ⱦ��������ɻ�����Ⱦ��(4)ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬��װ�õ�������û�����⣬��������ϩ����(��ͨ��Һ��)�ٶȹ��죬δ���ü����վʹ���ˮ��ͨ����Ҳ�����Ƿ�Ӧ��ȡ��ϩʱ�¶�û��Ѹ�ٴﵽ170��(��д�����²�����Ҳ����)��������ȡ����Ӧ�õ����Ѷ���ɡ�
CH2=CH2����H2O�� CH2=CH2��Br2 �� CH2BrCH2Br��(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������d��������,���ڲ��������岻�ܼ�ʱ�ų���ʹװ���е�����ѹǿ����ƿbˮ����½����������е�ˮ����������������������(3)����c��NaOH��Һ�������dz�ȥ��ϩ�д�������������(�������̼����������)����ֹ��Ⱦ��������ɻ�����Ⱦ��(4)ijѧ������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣬��װ�õ�������û�����⣬��������ϩ����(��ͨ��Һ��)�ٶȹ��죬δ���ü����վʹ���ˮ��ͨ����Ҳ�����Ƿ�Ӧ��ȡ��ϩʱ�¶�û��Ѹ�ٴﵽ170��(��д�����²�����Ҳ����)��������ȡ����Ӧ�õ����Ѷ���ɡ�
��ϰ��ϵ�д�
�����Ŀ

 2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4 ��2H2O
��2H2O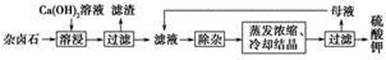
 ?
? CaCO3(s)��
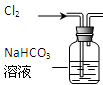
CaCO3(s)��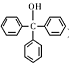 ����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��
����һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壬ʵ���Һϳ������״���������ͼ1��ʾ��װ����ͼ��ʾ��