��Ŀ����
18��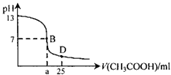 ��ͼΪ�����£���25mlijŨ�ȵ�NaOH��Һ����μ���0.2mol•L-1CH3COOH��Һ�Ĺ�������ҺpH�ı仯���ߣ�����˵������ȷ���ǣ�������
��ͼΪ�����£���25mlijŨ�ȵ�NaOH��Һ����μ���0.2mol•L-1CH3COOH��Һ�Ĺ�������ҺpH�ı仯���ߣ�����˵������ȷ���ǣ�������| A�� | NaOH��Һ��ʼŨ��Ϊ0.1mol•L-1 | |
| B�� | a��12.5 | |
| C�� | B����Һ��c��Na+��һ������c��CH3COO-�� | |
| D�� | D����Һ�У�c��Na+����c��CH3COO-����c��CH3COOH����c��H+����c��OH-�� |
���� A���������ǰ��Һ��pH=13���ݴ˼����NaOH��Һ��ʼŨ�ȣ�
B����a=12.5ʱ���������������ǡ�÷�Ӧ���ɴ����ƣ���Һ��ʾ���ԣ���Ϊ���ԣ������Ĵ���Ӧ���ٹ�����
C��B��ʱ��ҺΪ���ԣ���c��H+��=c��OH-�������ݵ���غ��֪��c��Na+��=c��CH3COO-����
D��D��Ϊ��Ũ�ȵĴ���ʹ����ƵĻ��Һ������ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ���c��CH3COO-����c��CH3COOH����c��H+����c��OH-�����ٽ�������غ��֪c��CH3COO-����c��Na+����
��� �⣺A������ͼ���֪���������ǰ��Һ��pH=13����NaOH��Һ��ʼŨ��Ϊ0.1mol/L����A��ȷ��
B����a=12.5ʱ��������������Ƶ����ʵ�����ȣ�����ǡ�÷�Ӧ���ɴ����ƣ���������Ӳ���ˮ�⣬��Һ��ʾ���ԣ���Ϊ���ԣ������Ĵ���Ӧ���Թ�������a��12.5����B��ȷ��
C��B��ʱ��Һ��pH=7����Һ�����ԣ���c��H+��=c��OH-�������ݵ���غ��֪��c��Na+��=c��CH3COO-������C��ȷ��
D��D��ʱ����25mL���ᣬ��ʱ��Һ������Ϊ��Ũ�ȵĴ���ʹ����ƵĻ��Һ������ĵ���̶ȴ��ڴ�������ӵ�ˮ��̶ȣ���c��CH3COO-����c��CH3COOH����c��H+����c��OH-�����ٽ�������غ��֪c��CH3COO-����c��Na+������Һ����ȷ������Ũ�ȴ�СΪ��c��CH3COO-����c��Na+����c��CH3COOH����c��H+����c��OH-������D����
��ѡD��
���� ���⿼��������ϵĶ����жϼ���ҺpH�ļ��㡢����Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷͼ���и�����������Ϊ���ؼ���ע��������Һ���������ҺpH�Ĺ�ϵ���ܹ����ݵ���غ㡢�����غ㼰�ε�ˮ��ԭ���ж���Һ�и�����Ũ�ȴ�С��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�| A�� | ��ϩ�������ӳɣ�1��2-�������飩 | |
| B�� | 1��3-����ϩ�����������ӳɣ����飩 | |
| C�� | �����뱽���Ȼ�����������Ӧ���ȱ��� | |
| D�� | �����ʵ����������������ڹ��������·�Ӧ�������飩 |
| A�� | �úϽ�����������Ϊ5.6g | |
| B�� | �Ͻ���ϡ���ᷴӦ������0.45mol H2 | |
| C�� | �úϽ��������������Ʒ�Ӧ��ת�Ƶĵ�����Ϊ0.6NA | |
| D�� | ���ӵ�ϡ�����к�0.6molH2SO4 |
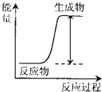
| A�� | H+H��H-H | |
| B�� | Mg+2HCl�TMgCl2+H2�� | |
| C�� | 2NH4Cl+Ba��OH��2•8H2O�TBaCl2+2NH3��+10H2O | |
| D�� | H2SO4+2KOH�TK2SO4+2H2O |

���з�����ȷ���ǣ�������
| A�� | 1molCPAE��NaOH��Һ��Ũ��ˮ��������Ӧʱ������ĵ�NaOH��Br2��H2�����ʵ����ֱ���3mol��4mol��7mol | |
| B�� | �����������������9��̼ԭ�ӹ�ƽ�� | |
| C�� | �����ᡢ���Ҵ���CPAE���ܷ���ȡ�����ӳɺ���ȥ��Ӧ | |
| D�� | ��FeCl3��Һ���Լ��������Ӧ���Ƿ���CPAE���� |
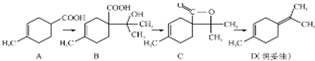 �����ͣ�D���������ϵ�ԭ�ϣ�������A�ϳɵõ���
�����ͣ�D���������ϵ�ԭ�ϣ�������A�ϳɵõ�������˵����ȷ���ǣ�������
| A�� | ��ϵͳ�������л���A������Ϊ3-��-3-����ϩ���� | |
| B�� | ����������ʣ�B���ܷ����Ӿ۷�Ӧ��Ҳ���ܷ������۷�Ӧ | |
| C�� | C �ķ���ʽΪC10H14O2 | |
| D�� | D ��Br2��CCl4��Һ��Ӧ�������4�������� |

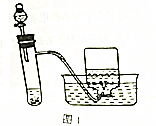
 �̷���FeSO4•7H2O���͵�����CuSO4•5H2O��������Ҫ�Ļ���ԭ�ϣ��ش��������⣺
�̷���FeSO4•7H2O���͵�����CuSO4•5H2O��������Ҫ�Ļ���ԭ�ϣ��ش��������⣺