ЬтФПФкШн
ЯТСаа№Ъіе§ШЗЕФЪЧЃЈЁЁЁЁЃЉЁЃ
| AЃЎдкГЃЮТЯТЃЌЮоТлЪЧДПЫЎЃЌЛЙЪЧЫсадЁЂМюадЛђжаадЯЁШмвКжаЃЌcЃЈHЃЋЃЉЁЄcЃЈOHЃЃЉЃН1ЁС10Ѓ14 |
| BЃЎcЃЈHЃЋЃЉЃН1ЁС10Ѓ7 molЁЄLЃ1ЕФШмвКвЛЖЈЪЧжаадШмвК |
| CЃЎ0.2 molЁЄLЃ1 CH3COOHШмвКжаЕФcЃЈHЃЋЃЉЪЧ0.1 molЁЄLЃ1 CH3COOHШмвКжаЕФcЃЈHЃЋЃЉЕФ2БЖ |
| DЃЎШЮКЮХЈЖШЕФШмвКЖМПЩвдгУpHРДБэЪОЦфЫсМюадЕФЧПШѕ |
A
НтЮі

СЗЯАВсЯЕСаД№АИ
 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
ЯрЙиЬтФП
ЯЁАБЫЎжаДцдкЯТЪіЦНКтЃК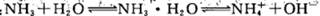 ИФБфЯТСаЬѕМўЃЌФмЪЙЕчРыГЬЖШдіДѓЕФЪЧ
ИФБфЯТСаЬѕМўЃЌФмЪЙЕчРыГЬЖШдіДѓЕФЪЧ
| AЃЎМгХЈАБЫЎ | BЃЎЩ§ИпЮТЖШ | CЃЎМгNH4ClШмвК | DЃЎМгNaOHШмвК |
ЪвЮТЯТЯђ10 mL pH=3ЕФДзЫсШмвКжаМгЫЎЯЁЪЭКѓЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
| AЃЎШмвКжаЕМЕчСЃзгЕФЪ§ФПМѕЩй |
BЃЎШмвКжа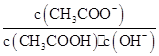 ВЛБф ВЛБф |
| CЃЎДзЫсЕФЕчРыГЬЖШдіДѓЃЌc(H+)врдіДѓ |
| DЃЎдйМгШы10 mL pH=11ЕФNaOHШмвКЃЌЛьКЯвКpH=7 |
ЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
| AЃЎГЃЮТЯТЃЌ0.1 mol/L NH4ClШмвКМгеєСѓЫЎЯЁЪЭЃЌШмвКЕФpHВЛЖЯдіДѓЃЌЕЋЮоЯоНгНќ7 |
| BЃЎ25 ЁцЪБЃЌШмвКжаЫЎЕчРыГіЕФc(HЃЋ)КЭЫЎЕчРыГіЕФc(OHЃ)ЕФГЫЛ§вЛЖЈЕШгк10Ѓ14 |
| CЃЎpHЃН6ЕФДПЫЎжаЃЌc(OHЃ)ЃН10Ѓ8 molЁЄLЃ1 |
| DЃЎЯЁЪЭ0.01 molЁЄLЃ1ЕФДзЫсШмвКЪБЃЌШмвКжаЫљгаЕФРызгХЈЖШОљМѕаЁ |
ГЃЮТЯТЃЌЯђ20 mL 0.2 mol/L H2AШмвКжаЕЮМг0.2 mol/L NaOHШмвКЁЃгаЙиСЃзгЕФЮяжЪЕФСПБфЛЏШчЭМ8Ѓ2(ЦфжаЂёДњБэH2AЃЌЂђДњБэHAЃЃЌЂѓДњБэA2Ѓ)ЃЌИљОнЭМЪОХаЖЯЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)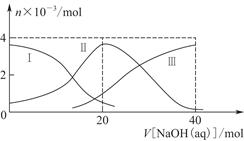
| AЃЎЕБV[NaOH(aq)]ЃН20 mLЪБЃЌШмвКжаРызгХЈЖШДѓаЁЙиЯЕЃКc(NaЃЋ)>c(HAЃ)>c(HЃЋ)>c(A2Ѓ)>c(OHЃ) |
| BЃЎЕШЬхЛ§ЕШХЈЖШЕФNaOHШмвКгыH2AШмвКЛьКЯКѓЃЌЦфШмвКжаЫЎЕФЕчРыГЬЖШБШДПЫЎДѓ |
| CЃЎH2AЕквЛВНЕчРыЕФЗНГЬЪНЮЊH2A=HAЃЃЋHЃЋ |
| DЃЎЯђNaHAШмвКМгШыЫЎЯЁЪЭЕФЙ§ГЬжаЃЌpHПЩФмдіДѓвВПЩФмМѕаЁ |
НёгаЪвЮТЯТЫФжжШмвК,гаЙиа№ЪіВЛе§ШЗЕФЪЧ(ЁЁ ЁЁ)
| | Ђй | Ђк | Ђл | Ђм |
| pH | 11 | 11 | 3 | 3 |
| ШмвК | АБЫЎ | ЧтбѕЛЏФЦШмвК | ДзЫс | бЮЫс |
A.ЂйЁЂЂкжаЗжБ№МгШыЪЪСПЕФТШЛЏяЇОЇЬхКѓ,СНШмвКЕФpHОљМѕаЁ
B.ЗжБ№МгЫЎЯЁЪЭ10БЖ,ЫФжжШмвКЕФpH:ЂйЃОЂкЃОЂмЃОЂл
C.ЂйЁЂЂмСНШмвКЕШЬхЛ§ЛьКЯ,ЫљЕУШмвКжаc(Cl-)ЃОc(N
 )ЃОc(OH-)ЃОc(H+)
)ЃОc(OH-)ЃОc(H+)D.Va L ЂмгыVb L ЂкШмвКЛьКЯ,ШєЛьКЯКѓШмвКpH=4,дђVaЁУVb=11ЁУ9
дк18 ЁцЪБЃЌH2SO3ЕФK1ЃН1.5ЁС10-2ЁЂK2ЃН5.0ЁС10-7ЃЌH2CO3ЕФK1ЃН1.4ЁС10-7ЁЂK2ЃН2.7ЁС10-11ЃЌдђЯТСаЫЕЗЈжае§ШЗЕФЪЧ ( )
| AЃЎЖрдЊШѕЫсЕФЫсаджївЊгЩЕквЛВНЕчРыОіЖЈЃЌбЧСђЫсЕФЫсадШѕгкЬМЫс |
| BЃЎЖрдЊШѕЫсЕФЫсаджївЊгЩЕкЖўВНЕчРыОіЖЈЃЌЬМЫсЕФЫсадШѕгкбЧСђЫс |
| CЃЎЖрдЊШѕЫсЕФЫсаджївЊгЩЕквЛВНЕчРыОіЖЈЃЌбЧСђЫсЕФЫсадЧПгкЬМЫс |
| DЃЎЖрдЊШѕЫсЕФЫсаджївЊгЩЕкЖўВНЕчРыОіЖЈЃЌЬМЫсЕФЫсадЧПгкбЧСђЫс |