��Ŀ����
��4 moI A�����2 mol B������2 L�������л�ϲ���һ�������·������·�Ӧ2A(g)��B(g)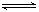 2C(g)������2�����C��Ũ��Ϊ0.6 mo1��L-1���������м���˵����
2C(g)������2�����C��Ũ��Ϊ0.6 mo1��L-1���������м���˵����
��������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.3 mol��L-1��s -1
��������B��ʾ�ķ�Ӧ��ƽ������Ϊ0.6 mol��L-1��s -1
�� 2sʱ����A��ת����Ϊ70%
�� 2sʱ����B��Ũ��Ϊ0.7 mo1��L-1
������ȷ����( )
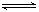 2C(g)������2�����C��Ũ��Ϊ0.6 mo1��L-1���������м���˵����
2C(g)������2�����C��Ũ��Ϊ0.6 mo1��L-1���������м���˵������������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.3 mol��L-1��s -1
��������B��ʾ�ķ�Ӧ��ƽ������Ϊ0.6 mol��L-1��s -1
�� 2sʱ����A��ת����Ϊ70%
�� 2sʱ����B��Ũ��Ϊ0.7 mo1��L-1
������ȷ����( )
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
B
��2 s���� C ��Ũ��Ϊ 0.6 mol��L��1 ��������C��1.2mol����������AB�ֱ���1.2mol��0.6mol����AB�ķ�Ӧ���ʷֱ���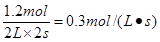 ��
�� ������ȷ���ڲ���ȷ��A��ת������1.2��4��100����30�����۲���ȷ��B��Ũ���ǣ�2mol��0.6mol����2L��0.7mol/L������ȷ����ѡB��
������ȷ���ڲ���ȷ��A��ת������1.2��4��100����30�����۲���ȷ��B��Ũ���ǣ�2mol��0.6mol����2L��0.7mol/L������ȷ����ѡB��
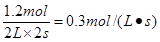 ��
�� ������ȷ���ڲ���ȷ��A��ת������1.2��4��100����30�����۲���ȷ��B��Ũ���ǣ�2mol��0.6mol����2L��0.7mol/L������ȷ����ѡB��
������ȷ���ڲ���ȷ��A��ת������1.2��4��100����30�����۲���ȷ��B��Ũ���ǣ�2mol��0.6mol����2L��0.7mol/L������ȷ����ѡB��
��ϰ��ϵ�д�
�����Ŀ
 pC(g)�ﵽƽ���ѹ�����������������A��ת������֮���͡�����˵���У���ȷ���ǣ� ��
pC(g)�ﵽƽ���ѹ�����������������A��ת������֮���͡�����˵���У���ȷ���ǣ� �� ?nC(��)��Q��һ�������´ﵽƽ��ı�������������ش�
?nC(��)��Q��һ�������´ﵽƽ��ı�������������ش� 2Z(g)��ӦӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ����������Y���������������������ȷ����(����)
2Z(g)��ӦӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ����������Y���������������������ȷ����(����)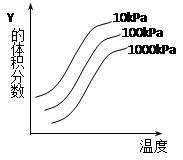
 pC (g) + qD (g)������5���Ӵﵽƽ�⣬��ʱ�����ʵı仯ΪA����Ϊa mol /L��B��ƽ����Ӧ����VB =" a/15" mol/ (L min)��C����2a / 3 mol/L����ʱ������ϵͳѹǿ������A��C�İٷֺ������䣬��m��n��p��qΪ�� ��
pC (g) + qD (g)������5���Ӵﵽƽ�⣬��ʱ�����ʵı仯ΪA����Ϊa mol /L��B��ƽ����Ӧ����VB =" a/15" mol/ (L min)��C����2a / 3 mol/L����ʱ������ϵͳѹǿ������A��C�İٷֺ������䣬��m��n��p��qΪ�� �� 2AB(g) �ﵽƽ��״̬�ı�־��
2AB(g) �ﵽƽ��״̬�ı�־�� CHCl3+ HCl���˷�Ӧ���и���Ӧ��������CH2Cl2��CH3Cl��CH4�ȡ���֪CCl4�ķе�Ϊ770C��CHCl3�ķе�Ϊ61.20C�����ܱ������У��÷�Ӧ�ﵽƽ�����������ݣ����費���Ǹ���Ӧ��
CHCl3+ HCl���˷�Ӧ���и���Ӧ��������CH2Cl2��CH3Cl��CH4�ȡ���֪CCl4�ķе�Ϊ770C��CHCl3�ķе�Ϊ61.20C�����ܱ������У��÷�Ӧ�ﵽƽ�����������ݣ����費���Ǹ���Ӧ�� 2AB(g)���ﵽƽ��ı�־���ǣ� ��
2AB(g)���ﵽƽ��ı�־���ǣ� ��