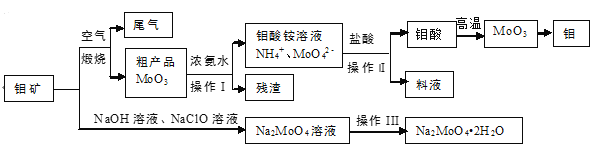
��Ŀ����
����Ŀ�������İ���ͭ����![]() ������ɱ�����ýȾ�����ڼ��Զ�ͭ��Ҳ���������Һ����Ҫ�ɷ֣��ڹ�ҵ����;�㷺�������¸���������ˮ���������Ҵ������ѣ��ڿ����в��ȶ�������ʱ�����ֽ⡣ij��ѧ��ȤС����Cu�ۡ�3mol/L�����ᡢŨ��ˮ��10% NaOH��Һ��95%���Ҵ���Һ��0.500 mol/Lϡ���ᡢ0.500 mol/L��NaOH��Һ���ϳ������İ���ͭ���岢�ⶨ�䴿�ȡ�
������ɱ�����ýȾ�����ڼ��Զ�ͭ��Ҳ���������Һ����Ҫ�ɷ֣��ڹ�ҵ����;�㷺�������¸���������ˮ���������Ҵ������ѣ��ڿ����в��ȶ�������ʱ�����ֽ⡣ij��ѧ��ȤС����Cu�ۡ�3mol/L�����ᡢŨ��ˮ��10% NaOH��Һ��95%���Ҵ���Һ��0.500 mol/Lϡ���ᡢ0.500 mol/L��NaOH��Һ���ϳ������İ���ͭ���岢�ⶨ�䴿�ȡ�
I��CuSO4��Һ���Ʊ�
�ٳ�ȡ4gͭ�ۣ���A����������10���Ӳ����Ͻ��裬������ȴ��
�����������м���30mL 3mol/L�����ᣬ��A�й��������������У����Ȳ����Ͻ��衣
�۳��ȹ��˵���ɫ��Һ��
(1)A����������Ϊ____��
(2)ijͬѧ��ʵ������1.5g��ͭ��ʣ�࣬��ͬѧ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���Խ�����ԭ��_____
II��������Ʊ�
�������Ʊ���CuSO4��Һ����ͼ��ʾ���в���
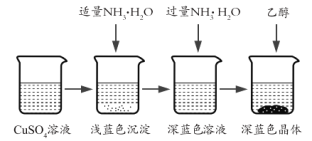
(3)��֪dz��ɫ�����ijɷ�Ϊ![]() ����д�����ɴ˳��������ӷ�Ӧ����ʽ_________��
����д�����ɴ˳��������ӷ�Ӧ����ʽ_________��
(4)��������ʱ���ü����Ҵ��ķ�����������Ũ���ᾧ��ԭ����__��
III���������IJⶨ
��ȷ��ȡmg���壬������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ���VmL10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������������ˮ��ϴ�����ڱڣ���V1mL C1mol/L���������Һ��ȫ���ա�ȡ�½���ƿ����C2mol/L NaOH����Һ�ζ���ʣ��HCl(ѡ�ü�����ָʾ��)�����յ�ʱ����V2mLNaOH��Һ��
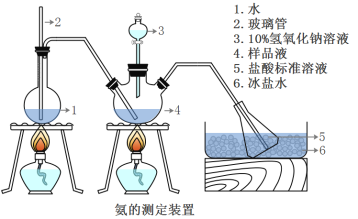
(5)Aװ���г������ܵ�����_____����Ʒ�а������������ı���ʽ_______��
(6)����ʵ���������ʹ�������ⶨ���ƫ�͵�ԭ����_______��
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ���
B������ʱ���ζ�ǰƽ�ӣ��ζ�����
C���ζ�������ѡ�÷�̪��ָʾ��
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڡ�
���𰸡����� ��Ӧ�������������Ũ�������У�ϡ�����Ũ��Ũ�������ˮ��ʹCuSO4��5H2Oʧȥ�ᾧˮ��ΪCuSO4 2Cu2++2NH3��H2O+SO42-=Cu2(OH)2SO4+2NH4+ Cu(NH3)4SO4��H2O�����������ȷֽ� ƽ����ѹ����ֹ�����͵��� ![]() BD
BD
��������
��1�����չ��壬Ӧ�������н��У���������AΪ�������ʴ�Ϊ��������
��2���õ���Ϊ����ͭ��������Һ��Ũ��ʱ�������Ũ��Ũ���������ˮ�ԣ�ʹCuSO4��5H2Oʧȥ�ᾧˮ��ΪCuSO4����ʹ�����Ϊ��ɫ���ʴ�Ϊ����Ӧ�������������Ũ�������У�ϡ�����Ũ��Ũ�������ˮ��ʹCuSO4��5H2Oʧȥ�ᾧˮ��ΪCuSO4��
��3��dz��ɫ�����ijɷ�ΪCu2(OH)2SO4������ԭ���غ��֪��Ӧ�����ӷ���ʽΪ2Cu2++2NH3��H2O+SO42-=Cu2(OH)2SO4+2NH4+���ʴ�Ϊ��2Cu2++2NH3��H2O+SO42-=Cu2(OH)2SO4+2NH4+��
��4����������ʱ���ü����Ҵ��ķ�����������Ũ���ᾧ��ԭ����Cu(NH3)4SO4��H2O�����������ȷֽ⣬�ʴ�Ϊ��Cu(NH3)4SO4��H2O�����������ȷֽ⡣
��5��Aװ���г������ܿ���ƽ����ѹ����ֹ�����͵��������ã��백����Ӧ��n(HCl)=10-3V1L��0.5mol/L-10-3V2L��0.5mol/L=0.5��10-3(V1-V2)mol�����ݰ�����HCl�Ĺ�ϵʽ��֪��n(NH3)=n(HCl)= 0.5��10-3(V1-V2)mol������Ʒ�а�����������Ϊ![]() ���ʴ�Ϊ��ƽ����ѹ����ֹ�����͵�����
���ʴ�Ϊ��ƽ����ѹ����ֹ�����͵�����![]() ��
��
��6������������ⶨ���ƫ�ߣ���V2ƫС��
A.�ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܣ�Ũ��ƫ�ͣ���V2ƫ����ƫ�ͣ���A����
B.����ʱ���ζ�ǰƽ�ӣ��ζ����ӣ�����V2ƫС������ƫ�ߣ���B��ȷ��
C.�ζ�������ѡ�÷�̪��ָʾ������ʵ��û��Ӱ�죬��C����
D.ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڣ���������ƫ�٣���Ҫ����������ƫ�٣���V2ƫС������ƫ�ߣ���D��ȷ��
�ʴ�Ϊ��BD��