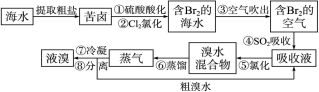
��Ŀ����
��һƿ�������Һ�����п�����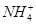 ��K����Na����Mg2����Ba2����Al3����Cl����I����
��K����Na����Mg2����Ba2����Al3����Cl����I���� ��
��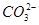 ��S2����
��S2����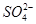 ��
�� ��
��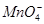 ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺
(1)ȡpH��ֽ���飬��Һ��ǿ���ԣ������ų� ���ӵĴ��ڣ�
(2)ȡ��������Һ����������CCl4������������ˮ������CCl4�����ɫ�������ų� ���ӵĴ��ڣ�
(3)��ȡ��������Һ��μ���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ���������������ֿ����ų� ���ӵĴ��ڣ�
(4)ȡ����������������Һ�μ�Na2CO3��Һ���а�ɫ�������ɣ�֤�� ���Ӵ��ڣ��ֿ��ų� ���ӵĴ��ڣ�
(5)��(3)�õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ��������������ʵ����ʵȷ��������Һ�п϶����ڵ������� ��������ȷ���Ƿ���ڵ������� ��
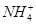 ��K����Na����Mg2����Ba2����Al3����Cl����I����
��K����Na����Mg2����Ba2����Al3����Cl����I���� ��
��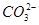 ��S2����
��S2����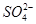 ��
�� ��
��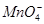 ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺(1)ȡpH��ֽ���飬��Һ��ǿ���ԣ������ų� ���ӵĴ��ڣ�
(2)ȡ��������Һ����������CCl4������������ˮ������CCl4�����ɫ�������ų� ���ӵĴ��ڣ�
(3)��ȡ��������Һ��μ���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ���������������ֿ����ų� ���ӵĴ��ڣ�
(4)ȡ����������������Һ�μ�Na2CO3��Һ���а�ɫ�������ɣ�֤�� ���Ӵ��ڣ��ֿ��ų� ���ӵĴ��ڣ�
(5)��(3)�õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ��������������ʵ����ʵȷ��������Һ�п϶����ڵ������� ��������ȷ���Ƿ���ڵ������� ��
(1)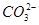 ��S2����
��S2����
(2) NO��
NO��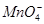
(3)Mg2����Al3��
(4)Ba2����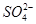
(5)I����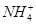 ��Ba2����K����Na����Cl��
��Ba2����K����Na����Cl��
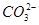 ��S2����
��S2����
(2)
 NO��
NO��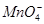
(3)Mg2����Al3��
(4)Ba2����
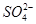
(5)I����
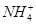 ��Ba2����K����Na����Cl��
��Ba2����K����Na����Cl��(1)��Һ��ǿ���ԣ������ų�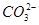 ��S2����
��S2���� ����������ӡ�(2)��������CCl4������������ˮ������CCl4�����ɫ��һ������I���������ų�
����������ӡ�(2)��������CCl4������������ˮ������CCl4�����ɫ��һ������I���������ų� ��
��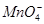 ��ǿ���������ӡ�(3)����NaOH��Һ�������к͵μ������Һ�������������϶�������Mg2����Al3����(4)������Һ��̼������Һ���а�ɫ�����������϶�����Ba2�����϶�������
��ǿ���������ӡ�(3)����NaOH��Һ�������к͵μ������Һ�������������϶�������Mg2����Al3����(4)������Һ��̼������Һ���а�ɫ�����������϶�����Ba2�����϶�������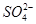 ��(5)��(3)��Һ���ȣ���ʹʪ��ĺ�ɫʯ����ֽ����������ų����϶�����
��(5)��(3)��Һ���ȣ���ʹʪ��ĺ�ɫʯ����ֽ����������ų����϶�����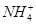 ���ۺ����ϣ��϶����ڵ�������I����
���ۺ����ϣ��϶����ڵ�������I����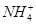 ��Ba2��������ȷ����������K����Na����Cl����
��Ba2��������ȷ����������K����Na����Cl����
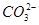 ��S2����
��S2���� ����������ӡ�(2)��������CCl4������������ˮ������CCl4�����ɫ��һ������I���������ų�
����������ӡ�(2)��������CCl4������������ˮ������CCl4�����ɫ��һ������I���������ų� ��
��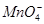 ��ǿ���������ӡ�(3)����NaOH��Һ�������к͵μ������Һ�������������϶�������Mg2����Al3����(4)������Һ��̼������Һ���а�ɫ�����������϶�����Ba2�����϶�������
��ǿ���������ӡ�(3)����NaOH��Һ�������к͵μ������Һ�������������϶�������Mg2����Al3����(4)������Һ��̼������Һ���а�ɫ�����������϶�����Ba2�����϶�������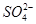 ��(5)��(3)��Һ���ȣ���ʹʪ��ĺ�ɫʯ����ֽ����������ų����϶�����
��(5)��(3)��Һ���ȣ���ʹʪ��ĺ�ɫʯ����ֽ����������ų����϶�����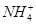 ���ۺ����ϣ��϶����ڵ�������I����
���ۺ����ϣ��϶����ڵ�������I����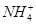 ��Ba2��������ȷ����������K����Na����Cl����
��Ba2��������ȷ����������K����Na����Cl����
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ