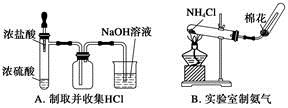
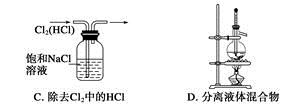
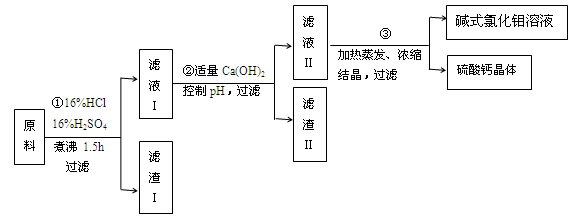
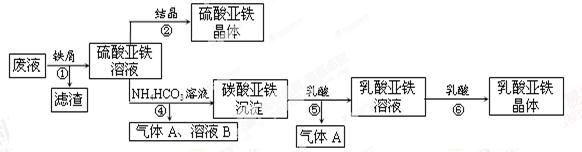
��Ŀ����
ij��ѧС���������������������װ�ã���ͼ�����Ի�����Ϊ��Ҫԭ���Ʊ�����ϩ��
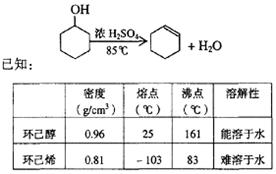
(1)�Ʊ���Ʒ
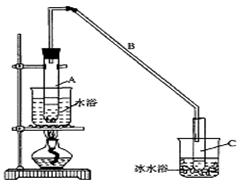
��12.5 mL�����������Թ�A�У��ټ���l mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ���(�g����f��)���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________________________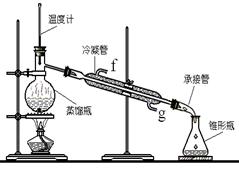


(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���l mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ���(�g����f��)���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________________________

��1���ٷ�ֹ���ҷ��� ���� ��ʹ����ϩҺ�������ٻӷ�
��2�����ϲ� C ��g ��83 ��
�Ʊ���Ʒʱ���������Ʒһ������
��2�����ϲ� C ��g ��83 ��
�Ʊ���Ʒʱ���������Ʒһ������
�����������1����A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�������������ʹ����ϩҺ����
�ڻ���ϩ���۷е�ͣ������Թ�C���ڱ�ˮԡ�е�Ŀ����ʹ����ϩҺ�������ٻӷ���
��2���ٻ���ϩ���ܶȱ�ˮС�����Լ��뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ㣻��������д�������ϴ�ӣ��ױ����������������������ʣ�������ϡH2SO4������ѡ��̼������Һ���������е��������ʷ�Ӧ����ѡC��
������ʱ��ȴˮ���¶˽����϶˳���������ȴˮ��g�ڽ�������ϩ�ķе���83�棬���Կ��Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ�����Ʊ���Ʒʱ���������Ʒһ��������ʹ���ʽ��͡�

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ
 ��K����Na����Mg2����Ba2����Al3����Cl����I����
��K����Na����Mg2����Ba2����Al3����Cl����I����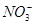 ��
��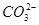 ��S2����
��S2����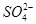 ��
��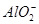 ��
��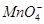 ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺