��Ŀ����
�����������ʵ���Ҫ���������ʽṹ����ش��������⡣
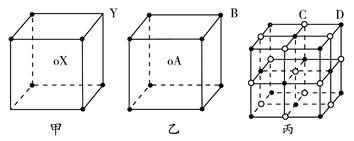
��1����ͼ��ʯī�Ľṹ���侧���д��ڵ��������� ������ţ� 
A���Ҽ� B���м� C����� D����λ�� E�����Ӽ������� F�������� G�����Ӽ�
��2�� ������ھ����˵������ȷ����___________
| A�������۵��ɵ͵��ߣ�CF4<CCl4<CBr4<CI4 |
| B��Ӳ���ɴ�С�����ʯ>̼����>����� |
| C���۵��ɸߵ��ͣ�Na>Mg>Al |
| D���������ɴ�С��NaF> NaCl> NaBr>NaI |

ͼ�� ͼ�� ͼ��
�� ͼI��ʾ�ľ�������Ca2+��������ҵȾ����Ca2+������Ϊ ��
ͼIII��δ��ŵ�Cuԭ���γɾ������Χ����ڵ�Cuԭ����Ϊ ��
�� H3BO3������Bԭ���ӻ���ʽ______ ; CNO-����״Ϊ____________;
�� ���־������۵�ߵ͵�˳��Ϊ (��ջ�ѧʽ)��
H3BO3���������ۻ�ʱ���˷�����֮��������Ϊ
��4�� ̼��ij�ֵ��ʵľ�������ͼ��ʾ��һ����������_____��̼ԭ�ӣ����þ�����ܶ�Ϊ�� g/cm3�������ӵ�������ֵΪNA�����������������̼ԭ��֮��ľ���Ϊ_ ____cm(�ô���ʽ��ʾ)

��1��ABEF��2�� C��3�� ��12 12 �� sp2 ֱ���� ��CaF2 > Cu >H3BO3���Ӽ�������������� �ͷ��»��� ����4�� 8�� 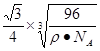
���������������1��ʯī�Ľṹ���侧���д��ڵ��������ЦҼ� �� �м� �� ���Ӽ�����������������2��A���⼸�־��嶼�Ƿ��Ӿ��塣���Ӽ��Է��Ӽ���������ϡ����ڽṹ���Ƶ�������˵����Է�������Խ���Ӽ���������Խ������۵㡢�е�Ҳ��Խ�ߡ���ȷ��B���⼸�־��嶼��ԭ�Ӿ��塣ԭ�Ӽ��Թ��ۼ���ϡ����ۼ�Խǿ���ƻ���ʹ�����ڻ����������ĵ�������Խ�ߣ�Ӳ�Ⱦ�Խ�����ۼ���ǿ����ԭ�Ӱ뾶�йأ�ԭ�Ӱ뾶ԽС�����ۼ�Խ�̣����ۼ���Խǿ��Ӳ��Ҳ��Խ��ԭ�Ӱ뾶Si>C������Si-Si>Si-C>C-C�������ɴ�С�����ʯ>̼����>����衣��ȷ��C��Na��Mg��Al���ǽ������塣���ڽ���������˵�����Ӱ뾶ԽС�������������Խ�࣬��������Խǿ�����Ӱ뾶Na+>Mg2+ >Al3+. �۵��ɵ͵��ߣ�Na>Mg>Al ������D�����Ƕ������Ӿ��塣��������ͬ�������Ӳ�ͬ�����Ӱ뾶ԽС�����Ӽ���Խǿ�������ܾ�Խ�����Ӱ뾶F-> Cl-> Br->I-�����Ծ������ɴ�С��NaF> NaCl> NaBr>NaI����ȷ�����ھ����˵������ȷ����C����3����ͼI ��ÿ����������Ca2+��������ҵȾ����Ca2+������Ϊ3����ͨ��ÿ��Ca2+���γ�8��������ÿ��Ca2+��������2�Σ�������Ca2+��������ҵȾ����Ca2+������Ϊ��8��3����2=12��. ͼIII�ɿ�����ÿ��Cuԭ����Χ��12��Cuԭ�ӣ�Cuԭ���γɾ������Χ����ڵ�Cuԭ����Ϊ12. �� H3BO3������Bԭ���ӻ���ʽsp2; CNO-����״Ϊֱ���͡��� ���־������۵�ߵ͵�˳��ΪCaF2 > Cu >H3BO3 H3BO3���������ۻ�ʱ���˷�����֮��������Ϊ�����֮��ķ��Ӽ����������»�������4������8��1/8=1������6��1/2=3������1��4=4.����ÿ�����о�������8��̼ԭ�ӡ����辧���ı߳�Ϊacm ���������Ϊa3 (cm) 3, ���������������̼ԭ��֮��ľ���Ϊ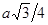 .
.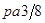 =12/NA.a3=(8��1/2)/pNA.����a=
=12/NA.a3=(8��1/2)/pNA.����a=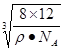 =
=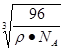 ;
;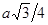 =
=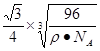 .
.
���㣺���龧������͡��۵㡢�е�ıȽϡ�����������Ŀ���������ļ����֪ʶ��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�������֣�ֻ��þ������̼����Ԫ�صľ��徹ȻҲ���г����ԣ���������Ԫ�ض��dz���Ԫ�أ��Ӷ�����㷺��ע�������ͳ��������һ��������ͼ��ʾ����þ���Ļ�ѧʽΪ
| A��Mg2CNi3 | B��MgCNi3 | C��MgCNi2 | D��MgC2Ni |
����˵����ȷ����
| A��Mg2+�İ뾶С��N3���İ뾶 |
| B��H2O��H2S�ȶ�����ΪH2O�д��ڷ��Ӽ���� |
| C��SiO2�����Է�����������CO2������SiO2���۵����CO2 |
| D����ԭ�ӹ��ɵľ���һ����ԭ�Ӿ��� |
[���ʽṹ������]��15�֣�
�±�Ϊ��ʽ���ڱ���һ���֣����е���Ŵ�����Ӧ��Ԫ�ء�
| �� | | | | | | | | | | | | | | | | | |
| | �� | | | | | | | | | | | | �� | �� | �� | �� | |
| | �� | | | | | | | | | | | | | | | �� | |
| | | | | | �� | | | | | �� | | | | | | | |
��2����Ԫ�آ�����γɵ�ˮ����������廯�����У�Ԫ�آ۵��ӻ���ʽΪ__________�ӻ���Ԫ�آ�����γɵĻ�����ľ���������__________��
��3��Ԫ�آܵĵ�һ������__________Ԫ�آݣ���д�������������������ĵ�һ�����ܣ�Ԫ�آ���Ԫ�آ��γɵ����X���ӵĿռ乹��Ϊ__________����д����Ԫ�آܵĵ��ʻ�Ϊ�ȵ�������ӡ����ӵĻ�ѧʽ__________����дһ�֣���
��4���ڲⶨԪ�آ�����γɻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ����_______��
��5��Ԫ�آܵ�����������Ӧ��ˮ����ϡ��Һ��Ԫ�آߵĵ��ʷ�Ӧʱ��Ԫ�آܱ���ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽΪ__________��
��6����������Xͨ�뺬��Ԫ�آ����ɫ��������Һ�У���Ӧ�����ӷ���ʽΪ__________��Ԫ�آ��ij��������ľ���ṹ��ͼ��ʾ������ʵ�����ʾԪ�آ�ԭ�ӣ���һ������������������ԭ����ĿΪ__________��

���з�Ӧ�У�������������ԭ��Ӧ��ͬʱ�������ȷ�Ӧ����
| A�����ȵ�̿��CO2��Ӧ | B��H2��O2��ȼ�շ�Ӧ |
| C������ϡ���ᷴӦ | D��Ba(OH)2��8H2O��NH4Cl��Ӧ |
���ھ�����Է��ԣ�����������ȷ���ǣ� ����
| A������ľ����ܹ��ڹ�̬ʱ�Զ���ɹ���Ķ����� |
| B��ȱ�ǵ��Ȼ��ƾ����ڱ��͵�NaCl��Һ��������Ϊ������������ |
| C��Բ�������н���ı���Բ�ε� |
| D���ɲ����Ƴɵ�Բ�εIJ����� |