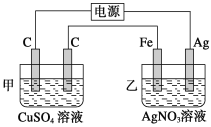
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥Μ·―ß–Υ»Λ–ΓΉι≤βΕ®Ρ≥Fe2(SO4)3―υΤΖ(÷ΜΚ§…ΌΝΩFeCl2‘”÷ )÷–Χζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΘ§Α¥“‘œ¬ Β―ι≤Ϋ÷ηΫχ––≤ΌΉςΘΚ
ΔΌ≥Τ»Γag―υΤΖΘ§÷Ο”Ύ…’±≠÷–ΘΜ
ΔΎΦ”»κ50 mL 1.0 mol/LœΓΝρΥαΚΆ“ΜΕ®ΝΩΒΡ’τΝσΥ°Θ§ Ι―υΤΖ»ήΫβΘ§»ΜΚσΉΦ»Ζ≈δ÷Τ≥…250.0 mL»ή“ΚΘΜ
ΔέΝΩ»Γ25.0 mL≤Ϋ÷ηΔΎ÷–≈δΒΟΒΡ»ή“ΚΘ§÷Ο”Ύ…’±≠÷–Θ§Φ”»κ ΝΩΒΡ¬»Υ°Θ§ ΙΖ¥”ΠΆξ»ΪΘΜ
ΔήΦ”»κΙΐΝΩΑ±Υ°Θ§≥δΖ÷ΫΝΑηΘ§ Ι≥ΝΒμΆξ»ΪΘΜ
ΔίΙΐ¬ΥΘ§œ¥Β”≥ΝΒμΘΜ
ΔόΫΪ≥ΝΒμΉΣ“ΤΒΫΡ≥»ίΤςΡΎΘ§Φ”»»ΓΔΫΝΑηΘ§÷±ΒΫΙΧΧε”…ΚλΚ÷…Ϊ»Ϊ≤Ω±δΈΣΚλΉΊ…ΪΚσΘ§‘ΎΗ…‘οΤς÷–ά以÷Ν “Έ¬ΚσΘ§≥ΤΝΩΘΜ
ΔΏΓ≠Γ≠

«κΗυΨί…œΟφ–π ωΘ§ΜΊ¥πΘΚ
Θ®1Θ©…œΆΦΥυ Ψ“«Τς÷–Θ§±Ψ Β―ι≤Ϋ÷ηΔΌΔΎΔέ÷–±Ί–κ”ΟΒΫΒΡ“«Τς”–…’±≠ΓΔΝΩΆ≤ΚΆ________(ΧνΉ÷ΡΗ)ΓΘ
Θ®2Θ©≤Ϋ÷ηΔΎ÷–ΘΚ≈δ÷Τ50 mL 1.0 mol/LœΓH2SO4–η“Σ98%(ΟήΕ»1.84 g/cm3)ΒΡ≈®H2SO4ΧεΜΐΈΣ________mLΘ§ΝΩ»ΓΗΟΧεΜΐΒΡ≈®H2SO4”ΟΒΫΝΩΆ≤ΙφΗώ «________ΓΘ
Θ®3Θ©―υΤΖ÷–ΒΡ‘”÷ Fe2ΘΪ”–Ϋœ«ΩΒΡΜΙ‘≠–‘Θ§Άξ≥…≤Δ≈δΤΫœ¬Ν–Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ
___Fe2ΘΪΘΪ___ClO2ΘΪ______=___Fe3ΘΪΘΪ___ClΓΣΘΪ___H2OΘ§¥”άμ¬έ…œΖ÷ΈωΘ§…œ ω Β―ι÷–»τΫΪ¬»Υ°ΗΡΈΣClO2 ±Θ§―θΜ·Β»ΝΩΒΡFe2ΘΪœϊΚΡClO2”κCl2ΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ________ΓΘ
Θ®4Θ©ΒΎΔό≤ΫΒΡ≤ΌΉς÷–Θ§ΫΪ≥ΝΒμΈοΉΣ“ΤΒΫ________Θ®Χν“«ΤςΟϊ≥ΤΘ©÷–Φ”»»Θ§≤Δ‘ΎΗ…‘οΤς÷–ά以ΒΫ “Έ¬Θ§≥ΤΝΩΤδ÷ ΝΩΓΘ
Θ®5Θ©»τ≤Ϋ÷ηΔό≤Μ‘ΎΗ…‘οΤς÷–ά以ȧ‘ρ≤βΕ®ΒΡΧζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΜα________(ΧνΓΑΤΪ¥σΓ±ΓΑΤΪ–ΓΓ±ΜρΓΑ≤Μ”ΑœλΓ±)ΘΜ»τ»ίΤς÷ ΝΩ «W1gΘ§Ήν÷’»ίΤςΚΆΙΧΧεΒΡΉή÷ ΝΩ «W2gΘ§‘ρ―υΤΖ÷–Χζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΈΣ_____(Ν–≥ωΥψ ΫΘ§≤Μ–ηΜ·Φρ)ΓΘ
ΓΨ¥πΑΗΓΩFG 2.7 10 mL 5 1 4 HΘΪ 5 1 2 2ΓΟ5 έαέω ΤΪ¥σ ![]() ΓΝ2ΓΝ56 g/molΓΝ
ΓΝ2ΓΝ56 g/molΓΝ![]() Γ¬agΓΝ100%
Γ¬agΓΝ100%
ΓΨΫβΈωΓΩ
≤βΕ®Ρ≥Fe2(SO4)3―υΤΖ(÷ΜΚ§…ΌΝΩFeCl2‘”÷ )÷–Χζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΘ§œ»Ά®Ιΐ―θΜ·ΦΝΫΪ―«Χζ―θΜ·≥…»ΐΦέΧζΘ§‘ΌΦ”Α±Υ°ΫΪFe3+±δΈΣ«β―θΜ·ΧζΘ§‘ΌΉΤ…’Ζ÷ΫβΒΟΒΫ―θΜ·ΧζΘ§”…―θΜ·ΧζΒΡ÷ ΝΩΦΤΥψΧζ‘ΣΥΊΒΡ÷ ΝΩΘ§ΉνΚσΒΟ≥ωΧζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΓΘ
ΘΚΘ®1Θ©≥ΤΝΩ“©ΤΖ”ΟΧλΤΫΘ§≈δ÷Τ“ΜΕ®Έο÷ ΒΡΝΩ≈®Ε»ΒΡ»ή“Κ”Ο»ίΝΩΤΩΘ§Ι ¥πΑΗΈΣΘΚFGΘΜ
Θ®2Θ©≈δ÷Τ50mL1.0mol/LœΓH2SO4–η“Σ98%Θ®ΟήΕ»1.84g/cm3Θ©ΒΡ≈®H2SO4ΧεΜΐΈΣ…ηΈΣVmLΘ§“άΨίœΓ Ά«ΑΚσ»ή“Κ»ή÷ Έο÷ ΒΡΝΩ≤Μ±δΘ§![]() =0.05LΓΝ1.0mol/LΘ§ΫβΒΟV=2.7Θ§ΝΩ»Γ ±”ΠΗΟ”Ο10mLΒΡΝΩΆ≤Θ§Ι ¥πΑΗΈΣΘΚ2.7Θ§10 mLΘΜ
=0.05LΓΝ1.0mol/LΘ§ΫβΒΟV=2.7Θ§ΝΩ»Γ ±”ΠΗΟ”Ο10mLΒΡΝΩΆ≤Θ§Ι ¥πΑΗΈΣΘΚ2.7Θ§10 mLΘΜ
Θ®3Θ©Ζ¥”Π÷–Θ§―«ΧζάκΉ”±δΜ·ΈΣΧζάκΉ”Θ§ClO2÷–¬»‘ΣΥΊΜ·ΚœΦέ¥”+4Φέ±δΜ·ΈΣ-1ΦέΘ§±δΜ·5ΦέΘ§ΒγΉ”ΉΣ“ΤΉν–ΓΙΪ±Ε ΐΈΣ5Θ§“άΨίΒγΉ” ΊΚψΓΔ‘≠Ή” ΊΚψΚΆΒγΚ… ΊΚψ≈δΤΫΒΟΒΫάκΉ”ΖΫ≥Χ ΫΈΣΘΚ5Fe2++ClO2+4 H+®T5Fe3++Cl-+2H2OΘΜ¥”άμ¬έ…œΖ÷ΈωΘ§…œ ω Β―ι÷–»τΫΪ¬»Υ°ΗΡΈΣClO2 ±Θ§Ά§ΝΩΜΙ‘≠–‘Έο÷ ±Μ―θΜ· ß»ΞΒγΉ”œύΆ§Θ§ ClO2ΓΪCl-ΓΪ5e-Θ§Cl2ΓΪ2Cl-ΓΪ2e-Θ§Β±ΒΟΒΫΒγΉ”œύΆ§ ±Θ§œϊΚΡClO2”κCl2ΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ2ΓΟ5Θ§Ι ¥πΑΗΈΣΘΚ5ΓΔ1ΓΔ4H+ΓΔ5ΓΔ1ΓΔ2ΘΜ 2ΘΚ5ΘΜ
Θ®4Θ©ΙΧΧεΈο÷ Φ”»»Ζ÷Ϋβ“ΜΑψΕΦ”Π‘Ύέαέω÷–Φ”»»Θ§Ι ¥πΑΗΈΣΘΚέαέωΘΜ
Θ®5Θ©»τ≤Ϋ÷ηΔό≤Μ‘ΎΗ…‘οΤς÷–ά以ȧΜαΈϋ ’Ω’Τχ÷–ΒΡΥ°’τΤχΘ§≥ΤΝΩΒΡ÷ ΝΩ‘ω¥σΘ§‘ρ≤βΕ®ΒΡΧζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΤΪ¥σΘΜ“ρΧζ‘ΣΥΊ÷ ΝΩ ΊΚψΘ§ Fe2O3÷–Χζ‘ΣΥΊΒΡ÷ ΝΩΈΣ![]() Θ§Εχ’β÷Μ «‘≠―υΤΖΒΡ °Ζ÷÷°“ΜΘ§―υΤΖ÷–Χζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐ «
Θ§Εχ’β÷Μ «‘≠―υΤΖΒΡ °Ζ÷÷°“ΜΘ§―υΤΖ÷–Χζ‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐ «![]() ΓΝ2ΓΝ56 g/molΓΝ
ΓΝ2ΓΝ56 g/molΓΝ![]() Γ¬agΓΝ100%ΘΜ
Γ¬agΓΝ100%ΘΜ
Ι ¥πΑΗΈΣΘΚΤΪ¥σΘΜ![]() ΓΝ2ΓΝ56 g/molΓΝ
ΓΝ2ΓΝ56 g/molΓΝ![]() Γ¬agΓΝ100%ΓΘ
Γ¬agΓΝ100%ΓΘ

 –¬ΩΈ±ξΫΉΧί‘ΡΕΝ―ΒΝΖœΒΝ–¥πΑΗ
–¬ΩΈ±ξΫΉΧί‘ΡΕΝ―ΒΝΖœΒΝ–¥πΑΗ ΩΎΥψ–ΡΥψΥΌΥψ”Π”ΟΧβœΒΝ–¥πΑΗ
ΩΎΥψ–ΡΥψΥΌΥψ”Π”ΟΧβœΒΝ–¥πΑΗ Ά§≤ΫΆΊ’Ι‘ΡΕΝœΒΝ–¥πΑΗ
Ά§≤ΫΆΊ’Ι‘ΡΕΝœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ≤ίΥαΨßΧεΒΡΉι≥…Ω…±μ ΨΈΣH2C2O4ΓΛxH2OΘ§ΈΣΝΥ≤βΕ®x÷ΒΘ§Ϋχ––œ¬ ω Β―ιΘΚ
ΔΌ≥Τ»Γn g≤ίΥαΨßΧε≈δ≥…100.00 mLΥ°»ή“ΚΘΜ
ΔΎ»Γ25.00 mLΥυ≈δ÷ΤΒΡ≤ίΥα»ή“Κ÷Ο”ΎΉΕ–ΈΤΩ÷–Θ§Φ”œΓΝρΥαΘ§”Ο≈®Ε»ΈΣa molΓΛLΘ≠1ΒΡKMnO4»ή“ΚΒΈΕ®Θ§
‘ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©–¥≥ωΒΈΕ® Β―ι÷–ΥυΖΔ…ζΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ_________________________________________________________
Θ®2Θ© Β―ι÷–KMnO4»ή“Κ”ΠΉΑ‘Ύ___________ ΫΒΈΕ®Ιή÷–Θ§ΒΈΕ®÷’ΒψΒΡ≈–Εœ“άΨί «_______________________________
Θ®3Θ©ΒΈΕ®Ιΐ≥Χ÷–”Ο»ΞV mL a molΓΛLΘ≠1ΒΡKMnO4»ή“ΚΘ§‘ρΥυ≈δ÷ΤΒΡ≤ίΥαΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ_______molΓΛLΘ≠1
Θ®4Θ©»τΒΈΕ®÷’ΒψΕΝ ΐ ±ΡΩΙβΗ© ”Θ§‘ρΦΤΥψ≥ωΒΡx÷ΒΩ…Ρή_______________Θ®ΧνΓΑΤΪ¥σΓ±ΓΔΓΑΤΪ–ΓΓ±ΓΔΓΑΈό”ΑœλΓ±Θ©
Θ®5Θ©≥ΝΒμΒΈΕ®®D®DΒΈΕ®ΦΝΚΆ±ΜΒΈΕ®ΈοΒΡ…ζ≥…Έο±»ΒΈΕ®ΦΝ”κ÷Η ΨΦΝΒΡ…ζ≥…ΈοΗϋΡ―»ήΓΘ
≤ΈΩΦœ¬±μ÷–ΒΡ ΐΨίΘ§»τ”ΟAgNO3ΒΈΕ®NaSCN»ή“ΚΘ§Ω…―Γ”ΟΒΡ÷Η ΨΦΝ «______(Χν―ΓœνΉ÷ΡΗ)ΓΘ
Ρ―»ήΈο | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
―’…Ϊ | ΑΉ | «≥ΜΤ | ΑΉ | Ή©Κλ | ΑΉ |
Ksp | 1.77ΓΝ10Θ≠10 | 5.35ΓΝ10Θ≠13 | 1.21ΓΝ10Θ≠16 | 1.12ΓΝ10Θ≠12 | 1.0ΓΝ10Θ≠12 |
AΘ°NaCl BΘ°NaBr CΘ°NaCN DΘ°Na2CrO4
ΓΨΧβΡΩΓΩ¬»ΤχΩ…”Ο”Ύ÷Τ»ΓΤ·ΑΉΦΝΚΆΉ‘ά¥Υ°œϊΕΨΓΘ
Θ®1Θ©ΫΪ¬»ΤχΆ®»κΥ°÷–÷ΤΒΟ¬»Υ°Θ§¬»Υ°Ω…”Ο”ΎΤ·ΑΉΘ§Τδ÷–ΤπΤ·ΑΉΉς”ΟΒΡΈο÷ «________Θ®Χν–¥Μ·―ß ΫΘ©ΓΘ
Θ®2Θ©ΓΑ84Γ±œϊΕΨ“Κ“≤Ω…”Ο”ΎΤ·ΑΉΘ§ΤδΙΛ“Β÷ΤΖ® «ΩΊ÷Τ‘Ύ≥ΘΈ¬ΧθΦΰœ¬Θ§ΫΪ¬»ΤχΆ®»κNaOH»ή“Κ÷–Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______________________ΓΘ
Θ®3Θ©Ά§―ßΟ«ΧΫΨΩΓΑ84Γ±œϊΕΨ“Κ‘Ύ≤ΜΆ§pHœ¬ ΙΚλ÷ΫΆ …ΪΒΡ«ιΩωΘ§ΉωΝΥ»γœ¬ Β―ιΘΚ
≤Ϋ÷η1ΘΚΫΪ5 mL – έΓΑ84Γ±œϊΕΨ“ΚœΓ Ά100±ΕΘ§≤βΒΟœΓ ΆΚσ»ή“ΚΒΡpH=12ΘΜ
≤Ϋ÷η2ΘΚΫΪœΓ ΆΚσ»ή“ΚΗς20 mLΖ÷±πΦ”»κ3ΗωΫύΨΜΒΡ–Γ…’±≠÷–ΘΜ
≤Ϋ÷η3ΘΚ”ΟH2SO4»ή“ΚΫΪ3Ηω…’±≠ΡΎ»ή“ΚΒΡpHΖ÷±πΒς÷Ν10ΓΔ7ΚΆ4ΓΘΘ®»ή“ΚΧεΜΐ±δΜ·Κω¬‘≤ΜΦΤΘ©
≤Ϋ÷η4ΘΚ‘Ύ3Ηω…’±≠÷–Ζ÷±πΖ≈»κ¥σ–ΓœύΆ§ΒΡΚλ÷ΫΘ§Ιέ≤λœ÷œσΘ§Φ«¬Φ»γœ¬ΘΚ
…’±≠ | »ή“ΚΒΡpH | œ÷œσ |
a | 10 | 10 minΚσΘ§Κλ÷ΫΜυ±Ψ≤ΜΆ …ΪΘΜ4 hΚσΚλ÷ΫΆ …Ϊ |
b | 7 | 10 minΚσΘ§Κλ÷Ϋ―’…Ϊ±δ«≥ΘΜ4 hΚσΚλ÷ΫΆ …Ϊ |
c | 4 | 10 minΚσΘ§Κλ÷Ϋ―’…Ϊ±δΒΟΗϋ«≥ΘΜ4 hΚσΚλ÷ΫΆ …Ϊ |
“―÷ΣΘ§»ή“Κ÷–Cl2ΓΔHClOΚΆClO-Έο÷ ΒΡΝΩΖ÷ ΐΘ®ΠΝΘ©Υφ»ή“ΚpH±δΜ·ΒΡΙΊœΒ»γœ¬ΆΦΥυ ΨΘΚ
![]()

ΔΌ”… Β―ιœ÷œσΩ…ΜώΒΟ“‘œ¬Ϋα¬έΘΚ»ή“ΚΒΡpH‘Ύ4ΓΪ10ΖΕΈßΡΎΘ§pH‘Ϋ¥σΘ§Κλ÷ΫΆ …Ϊ________ΓΘ
ΔΎΫαΚœΆΦœώΫχ––Ζ÷ΈωΘ§bΓΔcΝΫ…’±≠÷– Β―ιœ÷œσ≥ωœ÷≤ν“λΒΡ‘≠“ρ «________ΓΘ
Θ®4Θ©”…”Ύ¬»ΤχΜα”κΉ‘ά¥Υ°÷–ΒΡ”–ΜζΈοΖΔ…ζΖ¥”Π…ζ≥…Ε‘»ΥΧε”–ΚΠΒΡΈο÷ Θ§»ΥΟ«≥Δ ‘―–ΨΩ≤Δ Ι”Ο–¬ΒΡΉ‘ά¥Υ°œϊΕΨΦΝΘ§»γClO2ΤχΧεΨΆ «“Μ÷÷–¬–ΆΗΏ–ßΚ§¬»œϊΕΨΦΝΓΘ
ΔΌ“Μ÷÷÷Τ±ΗClO2ΒΡΖΫΖ® «”ΟSO2Ά®»κΝρΥαΥαΜ·ΒΡNaClO3»ή“Κ÷–Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ________ΓΘ
ΔΎΝμ“Μ÷÷÷Τ±ΗClO2ΒΡΖΫΖ® «”ΟNaClO3”κ―ΈΥαΖ¥”ΠΘ§Ά§ ±”–Cl2…ζ≥…Θ§≤ζΈο÷–Cl2ΧεΜΐ‘Φ’Φ1/3Θ§ΟΩ…ζ≥…0.5 mol ClO2Θ§ΉΣ“Τ________mol e-ΓΘ
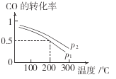
ΓΨΧβΡΩΓΩ“―÷ΣΘΚ![]() ±
±
Μ·―ß Ϋ |
|
|
|
ΒγάκΤΫΚβ≥Θ ΐ |
|
|
|
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ « ( )
A. ¥ΉΥαœΓ ΆΙΐ≥Χ÷–Θ§![]() ÷πΫΞΦθ–Γ
÷πΫΞΦθ–Γ
B. ![]() »ή“Κ÷–ΘΚ
»ή“Κ÷–ΘΚ![]()
C. œρ¥ΉΥαΜρHCN»ή“Κ÷–Φ”»κ![]() ,Ψυ≤ζ…ζ
,Ψυ≤ζ…ζ![]()
D. Έο÷ ΒΡΝΩ≈®Ε»œύΆ§ ±![]()