جâؤ؟ؤعبف
،¾جâؤ؟،؟I،¢تµرéتزسأ50 mL 0.50 mol،¤L£1رخثل،¢50 mL 0.55 mol،¤L£1 NaOHبـز؛؛حبçح¼ثùت¾×°ضأ£¬½ّذذ²â¶¨ضذ؛حببµؤتµر飬µأµ½±يضذµؤت¾ف£؛حê³ةدآءذختجâ£؛
تµرé´خت | ئًت¼خآ¶بt1/،و | ضصض¹خآ¶بt2/،و | |
رخثل | NaOHبـز؛ | ||
1 | 20.2 | 20.3 | 23.7 |
2 | 20.3 | 20.5 | 23.8 |
3 | 21.5 | 21.6 | 24.9 |

(1)²»ؤـسأجْث؟½ء°è°ô´ْجو»·ذخ²£ء§½ء°è°ôµؤہيسةتا_______
(2)¾ت¾ف´¦ہي£¬²âµأضذ؛حببخھ56.8 kJ،¤mol£1ئنبب»¯ر§·½³جت½خھ£؛___________
II£®تµرéتزضئ±¸دُ»ù±½تµرé×°ضأ؛ح²½ضèبçدآ£؛(تµرé²½ضè)

ب،100 mLةص±£¬سأ20 mLإ¨ءٍثلسë18 mLإ¨دُثلإنضئ»ى؛دز؛£¬½«»ى؛دثلذ،ذؤ¼سبëBضذ£¬°ر18 mL(15.84 g)±½¼سبëAضذ،£دٍتزخآدآµؤ±½ضذضًµخ¼سبë»ىثل£¬±كµخ±ك½ء°è£¬»ى؛د¾ùشب£¬شع50،«60،ودآ·¢ةْ·´س¦£¬ض±ضء·´س¦½لتّ،£
½«·´س¦ز؛ہنب´ضءتزخآ؛َµ¹بë·ضز؛آ©¶·ضذ£¬زہ´خسأةظء؟ث®،¢5% NaOHبـز؛؛حث®د´µس،£·ض³ِµؤ²ْخï¼سبëخقث®CaCl2؟إء££¬¾²ضأئ¬؟ج£¬ئْب¥CaCl2£¬½ّذذصôءَ´؟»¯£¬تص¼¯205،«210 ،وءَ·ض£¬µأµ½´؟دُ»ù±½18.00 g،£اë»ط´ً£؛
(1)×°ضأBµؤأû³ئتا________£¬×°ضأCµؤ×÷سأتا________________،£
(2)تµرéتزضئ±¸دُ»ù±½µؤ»¯ر§·½³جت½تا____________________________،£
(3)خھءثت¹·´س¦شع50،«60 ،ودآ½ّذذ£¬³£سأµؤ·½·¨تا__________،£
(4)شعد´µس²ظ×÷ضذ£¬µع¶´خث®د´µؤ×÷سأتا____________________،£
،¾´ً°¸،؟جْث؟´«بب؟ى£¬ببء؟ثًت§´َاززھسëرخثل·´س¦ H£«(aq)£«OH£(aq) =H2O(l)£»¦¤H£½£56.8kJ/mol ·ضز؛آ©¶· ہنؤ»طء÷،¢ئ½؛âئّر¹ 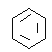 +HNO3
+HNO3![]()
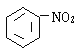 +H2O ث®ش،¼سبب د´ب¥²ذءôµؤNaOH¼°ةْ³ةµؤرخ
+H2O ث®ش،¼سبب د´ب¥²ذءôµؤNaOH¼°ةْ³ةµؤرخ
،¾½âخِ،؟
I£®(1)²»ؤـ½«»·ذخ²£ء§½ء°è°ô¸ؤخھحث؟½ء°è°ô£¬زٍخھحث؟½ء°è°ôتاببµؤء¼µ¼جه£¬½ًتôµ¼بب؟ى£¬ببء؟ثًت§¶à£»
(2)¾ت¾ف´¦ہي£¬²âµأضذ؛حببخھ56.8 kJ،¤mol£1£¬¼´د،رخثلسëد،اâرُ»¯ؤئبـز؛حêب«·´س¦ةْ³ة1molث®ت±·إ³ِµؤببء؟خھ56.8 kJ،¤mol£1£¬ضذ؛ح·´س¦µؤتµضتخھاâہë×س؛حاâرُ¸ùہë×س·´س¦ةْ³ةث®£¬شٍبب»¯ر§·½³جت½خھ£؛H£«(aq)£«OH£(aq) =H2O(l)£»¦¤H£½£56.8kJ/mol£»
II£®(1)¸ù¾فح¼ت¾£¬×°ضأBµؤأû³ئتا·ضز؛آ©¶·£»×°ضأAضذ±½،¢دُثلµبز×»س·¢£¬بفز×ثًت§£¬أـ±صجُ¼دآ»لµ¼ضآ×°ضأؤعر¹ا؟شِ´َ£¬×°ضأCخھہنؤ¹ـ£¬ئًہنؤ»طء÷،¢ئ½؛âئّر¹×÷سأ£»
(2)تµرéتزسأ±½سëإ¨دُثلشعإ¨ءٍثل،¢¼سببجُ¼دآةْ³ةدُ»ù±½سëث®£¬·´س¦·½³جت½خھ£؛ +HNO3
+HNO3![]()
 +H2O£»
+H2O£»
(3)خھءثت¹·´س¦شع50،«60 ،ودآ½ّذذ£¬·´س¦خآ¶بµحسعث®µؤ·ذµم£¬³£سأµؤ·½·¨تاث®ش،¼سبب£»
(4)شعد´µس²ظ×÷ضذ£¬µع¶´خث®د´µؤ×÷سأتاد´ب¥²ذءôµؤNaOH¼°ةْ³ةµؤرخ،£

 سإزيذ،°ïتضح¬²½؟عثمدµءذ´ً°¸
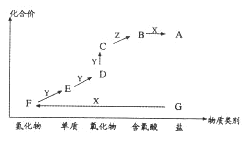
سإزيذ،°ïتضح¬²½؟عثمدµءذ´ً°¸،¾جâؤ؟،؟ءٍثلأ¾شعز½ءئةد¾كسذصٍ¾²،¢؟¹آخµب¹¦ذ§،£زشءâأ¾؟َ£¨ض÷زھ³ة·ضتاMgCO3£©خھض÷زھشءدضئ±¸ءٍثلأ¾µؤ·½·¨بçدآ£؛

£¨1£©²½ضè¢عضذ·¢ةْ·´س¦µؤہë×س·½³جت½خھ____________________________________،£
£¨2£©²½ضè¢ـضذµ÷½عpH=6.0~6.5µؤؤ؟µؤتا___________________________________،£
£¨3£©²½ضè¢فµؤ²ظ×÷خھ______________________________
£¨4£©زرضھثل¼îض¸ت¾¼ء°ظہï·سہ¶±نة«µؤpH·¶خ§بç±يثùت¾£؛
pH | < 8.0 | 8.0 ~ 9.6 | > 9.6 |
رصة« | »ئة« | آجة« | ہ¶ة« |
25،وت±£¬دٍMg(OH)2µؤ±¥؛حبـز؛ضذµخ¼س2µخ°ظہï·سہ¶ض¸ت¾¼ء£¬بـز؛ثù³تدضµؤرصة«خھ_____[25،وت±£¬Ksp[Mg(OH)2] =5.6،ء10-12]،£
،¾جâؤ؟،؟I،¢£¨1£©ئصح¨ذ؟أج¸ةµç³طµؤ½ل¹¹بçح¼ثùت¾،£»ط´ًدآءذختجâ،£

¢ظµç³طضذµç½âضتبـز؛خھ________،£
¢ع¸؛¼«·´س¦ت½خھ______________________________________،£
¢غ·إµçت±NH![]() دٍ________(جî،°ص¼«،±»ٍ،°¸؛¼«،±)زئ¶¯،£
دٍ________(جî،°ص¼«،±»ٍ،°¸؛¼«،±)زئ¶¯،£
£¨2£©·دµç³طضذµؤذ؟ئ¤³£سأسعتµرéتزضئاâئّ£¬·دذ؟ئ¤؛ح´؟ذ؟ء£·ض±ًسëح¬إ¨¶بµؤد،ءٍثل·´س¦£¬²ْةْاâئّثظآت½د´َµؤتا________£¬بôسأ¹ء؟µؤ´؟ذ؟ء£سëز»¶¨ء؟µؤد،ءٍثل·´س¦£¬خھءث¼س؟ى·´س¦ثظآتسض²»س°دى²ْةْاâئّµؤء؟£¬دآءذ´ëت©؟ةذذµؤتا________(جîذٍ؛إ)،£
A£®خ¢بب B£®¼سبëتتء؟رُ»¯ح C£®¼سبëةظء؟ءٍثلحبـز؛
D£®¼سث® E£®¼سبëةظء؟اâرُ»¯±µبـز؛
II،¢ز»¶¨جُ¼دآ£¬H2O2شعث®بـز؛ضذ·¢ةْ·ض½â·´س¦£؛2H2O2![]() 2H2O£«O2،ü£¬·´س¦¹³جضذ£¬²âµأ²»ح¬ت±¼نH2O2µؤخïضتµؤء؟إ¨¶ببçدآ±ي£؛
2H2O£«O2،ü£¬·´س¦¹³جضذ£¬²âµأ²»ح¬ت±¼نH2O2µؤخïضتµؤء؟إ¨¶ببçدآ±ي£؛
t/min | 0 | 20 | 40 | 60 | 80 |
c(H2O2)/(mol/L) | 0.80 | 0.40 | 0.20 | 0.10 | 0.05 |
£¨1£©H2O2µؤ·ض½â·´س¦________رُ»¯»¹ش·´س¦(جî،°تا،±»ٍ،°²»تا،±)،£
£¨2£©¸أ·ض½â·´س¦0،«20 minµؤئ½¾ù·´س¦ثظآتv(H2O2)خھ________ mol/(L،¤min)،£
£¨3£© ¼سبë0.1 molµؤMnO2·غؤ©سع50 mL¹رُ»¯اâµؤبـز؛ضذ(أـ¶بخھ1.1 g/mL)£¬شع±ê×¼×´؟ِدآ·إ³ِئّجهµؤجه»؛حت±¼نµؤ¹طدµبçح¼ثùت¾£¬·´س¦·إ³ِ![]() ئّجهثùذèزھµؤت±¼نخھ________£»A،¢B،¢C،¢Dثؤµم»¯ر§·´س¦ثظآت؟ىآµؤث³ذٍخھ________،£
ئّجهثùذèزھµؤت±¼نخھ________£»A،¢B،¢C،¢Dثؤµم»¯ر§·´س¦ثظآت؟ىآµؤث³ذٍخھ________،£

£¨4£©سة؛د³ةئّ(×é³ةخھH2،¢CO،¢؛حةظء؟CO2)ض±½سضئ±¸¶¼×أر£¬ئنضذض÷زھ¹³ج°üہ¨زشدآثؤ¸ِ·´س¦£؛
¼×´¼؛د³ة·´س¦£؛¢ظCO(g)£« 2H2(g)=CH3OH(g) ¦¤H1£½£90.1 kJ،¤mol£1
¢عCO2(g)£« 3H2(g)=CH3OH(g)£«H2O(g) ¦¤H2£½£49.0 kJ،¤mol£1
ث®أ؛ئّ±ن»»·´س¦£؛¢غCO(g) £« H2O (g)=CO2(g)£«H2(g) ¦¤H3£½£41.1 kJ،¤mol£1
¶¼×أر؛د³ة·´س¦£؛
سةH2؛حCOض±½سضئ±¸¶¼×أر(ءيز»²ْخïخھث®صôئّ)µؤبب»¯ر§·½³جت½خھ________________
،¾جâؤ؟،؟´¼حرث®تا؛د³ةد©جµؤ³£سأ·½·¨£¬تµرéتز؛د³ة»·¼؛د©µؤ·´س¦؛حتµرé×°ضأبçدآ£؛


؟ةؤـسأµ½µؤسذ¹طت¾فبçدآ£؛
دà¶ش·ض×سضتء؟ | أـ¶ب/£¨g،¤cm-3£© | ·ذµم/،و | بـ½âذش | |
»·¼؛´¼ | 100 | 0.9618 | 161 | خ¢بـسعث® |
»·¼؛د© | 82 | 0.8102 | 83 | ؤربـسعث® |
؛د³ة·´س¦£؛شعaضذ¼سبë20g»·¼؛´¼؛ح2ذ،ئ¬ثé´ةئ¬£¬ہنب´½ء¶¯دآآآ¼سبë1mLإ¨H2SO4£¬bضذح¨بëہنب´ث®؛َ£¬؟ھت¼»؛آ¼سببa£¬؟طضئءَ³ِخïµؤخآ¶ب²»³¬¹90،و،£
·ضہëجل´؟£؛·´س¦´ض²ْخïµ¹بë·ضز؛آ©¶·ضذ·ض±ًسأةظء؟5%ج¼ثلؤئبـز؛؛حث®د´µس£¬·ضہë؛َ¼سبëخقث®آب»¯¸ئ؟إء££¬¾²ضأز»¶خت±¼ن؛َئْب¥آب»¯¸ئ£¬×îضصح¨¹صôءَµأµ½´؟¾»»·¼؛د©10g،£»ط´ًدآءذختجâ£؛
£¨1£©×°ضأbµؤأû³ئتا__،£
£¨2£©¼سببز»¶خت±¼ن؛َ·¢دضحü¼ا¼س´ةئ¬£¬س¦¸أ²ةب،µؤصب·²ظ×÷تا__،£
A.ء¢¼´²¹¼س B.ہنب´؛َ²¹¼س C.²»ذè²¹¼س D.ضطذآإنءد
£¨3£©±¾تµرéضذ×îبفزײْةْµؤ¸±²ْخïµؤ½ل¹¹¼ٍت½خھ__،£
£¨4£©·ضہëجل´؟¹³جضذ¼سبëخقث®آب»¯¸ئµؤؤ؟µؤتا__،£
£¨5£©±¾تµرéثùµأµ½µؤ»·¼؛د©²ْآتتا__£¨جîصب·´ً°¸±ê؛إ£©،£
A.41% B.50% C.61% D.70%
،¾جâؤ؟،؟ؤ³ح¬ر§½ّذذس°دى²فثلبـز؛سë¸كأجثل¼طثلذشبـز؛·´س¦ثظآتزٍثطµؤرذ¾؟،£تزخآدآ£¬تµرéت¾فبçدآ£؛
تµرéذٍ؛إ | ¢ظ | ¢ع | ¢غ |
¼سبëتش¼ء | 4mL 0.01mol/L KMnO4 2mL 0.1mol/L H2C2O4 | 4mL 0.01mol/L KMnO4 2mL 0.1mol/L H2C2O4 ةظء؟MnSO4¹ججه | 4mL 0.01mol/L KMnO4 2mL 0.1mol/L H2C2O4 ةظء؟Na2SO4¹ججه |
حتة«ت±¼ن/s | 116 | 6 | 117 |
£¨1£©²فثلبـز؛سë¸كأجثل¼طثلذشبـز؛·´س¦µؤہë×س·½³جت½خھ___________________،£
£¨2£©¸أتµرé½لآغتا_______________________،£
£¨3£©½ّذذةدتِب¸ِتµرé؛َ£¬¸أح¬ر§½ّذذ·´ث¼£¬بدخھتµرé¢ظµؤدضدَ؟ةزشض¤أ÷ةدتِ½لآغ،£اëذ´³ِتµرé¢ظµؤدضدَخھ____________________،£
£¨4£©تµرé¢عر،سأMnSO4¹ججه¶ّ²»تاMnCl2¹ججهµؤشزٍتا_________________،£
£¨5£©¸أح¬ر§ؤâ²ةسأبçدآح¼ثùت¾µؤتµرé·½°¸¼جذّج½¾؟حâ½çجُ¼¶ش·´س¦ثظآتµؤس°دى،£

a£®¸أح¬ر§ؤâرذ¾؟µؤس°دىزٍثطتا___________________،£
b£®ؤمبدخھ¸أح¬ر§µؤتµرé·½°¸_______________£¨جî،°؛دہي،±»ٍ،°²»؛دہي،±£©£¬ہيسةتا____________________________،£