��Ŀ����
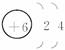
��X��Y��Z��W���ֶ�����Ԫ�أ�ԭ��������������X�������Ӿ���һ�����ӡ�Z��W�����ڱ��ڴ�������λ�ã����ǵĵ�����ͨ��״���¾�Ϊ��ɫ���塣Yԭ�ӵ������������Ǵ�����������2������ش���1��Z2�ĵ���ʽΪ______________��Y��ԭ�ӽṹʾ��ͼΪ______________��
��2����X2��W2����ͼ��ʾͨ��ijȼ�ϵ���У����У�b�缫Ϊ______________��(���������)���缫��ӦʽΪ____________________________��
��3��X��Y����Ԫ�����һ�����壬�ڱ�״�����ܶ�Ϊ
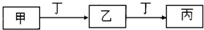
��4����X��Y��Z��W����Ԫ����ɵ���ʽ�Σ���������NaOH��Һ�ڼ��������·�Ӧ�����ӷ���ʽΪ_____________________________________________________________________��
��1����N����N�� 
��2���� O2+2H2O+4e-====4OH-
��3��C2H5OH![]() CH2=CH2��+H2O
CH2=CH2��+H2O
��4��![]() +
+![]() +2OH-
+2OH-![]() NH3��+2H2O+
NH3��+2H2O+![]() ���𰸺������ɣ�
���𰸺������ɣ�
����������һ�����ӵ�������ֻ��H+,�ɴ˿�֪X��HԪ�ء�����Y�Ĵ�������������֪�������������2ʱ��������ʱY��������������4��Ӧ��C���������У�ԭ��������C���ҵ��ʾ�Ϊ��ɫ�����������Ԫ��ֻ��N��O����Wԭ����������Z����֪WΪO��ZΪN��(2)���У�B��ͨ��������O2�����Ƿ�����ԭ��Ӧ����������Ӧ�缫��ӦʽΪO2+2H2O+4e-![]() 4OH-��(3)���У���״�����ܶ�Ϊ
4OH-��(3)���У���״�����ܶ�Ϊ![]() CH2=CH2��+H2O��(4)���У���C��H��N��O��ɵ���ʽ��ΪNH4HCO3������NaOH��Һ�����ȷ�����Ӧ�����ӷ���ʽΪ
CH2=CH2��+H2O��(4)���У���C��H��N��O��ɵ���ʽ��ΪNH4HCO3������NaOH��Һ�����ȷ�����Ӧ�����ӷ���ʽΪ![]() +
+![]() +2OH-====NH3��+2H2O+
+2OH-====NH3��+2H2O+![]() ��
��


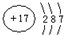

 ��X��Y��Z��W���ֶ�����Ԫ�أ�ԭ��������������X�������Ӿ���һ�����ӣ�Z��W�����ڱ��ڴ���ͬһ��������λ�ã����ǵĵ�����ͨ��״���¾�Ϊ��ɫ���壮Yԭ�ӵ������������Ǵ�����������2������ش�
��X��Y��Z��W���ֶ�����Ԫ�أ�ԭ��������������X�������Ӿ���һ�����ӣ�Z��W�����ڱ��ڴ���ͬһ��������λ�ã����ǵĵ�����ͨ��״���¾�Ϊ��ɫ���壮Yԭ�ӵ������������Ǵ�����������2������ش�