��Ŀ����
����Ŀ������˵����ȷ����
A. ����ʱ��0.1mol��L-1��������Һ��NH4Al(SO4)2 ��NH4Cl ��NH3��H2O ��CH3COONH4 �У�c (NH4+)�ɴ�С��˳���ǣ���>��>��>��
B. 0.1mol��L-1 NaHCO3��Һ�У�c(Na+)= 2c(CO32-)+ c(HCO3-)+c(H2CO3)
C. ����ʱ��pH=2��CH3COOH��Һ��HCl��Һ��pH=12�İ�ˮ��NaOH��Һ��������Һ����ˮ�����c(H+)�����
D. ����ʱ��0.3 mol��L-1HY��Һ��0.3 mol��L-1NaOH��Һ�������Ϻ���Һ��pH=9��������Һ��c(H+) +c(HY)= 1��10-5 mol��L-1
���𰸡�D
��������ͬŨ�ȵ�������Һ: ��NH4Al(SO4)2 ��NH4Cl ��NH3��H2O ��CH3COONH4 ,��(1)��������ˮ������笠����ӵ�ˮ��; (2)��笠�����ˮ��; (3)���������,�ҵ���ij̶Ⱥ���; (4)���������ˮ��ٽ�笠�����ˮ��,��c (NH4+)�ɴ�С��˳����: (1) >(2)> (4)> (3) ����A�Ǵ����; B. ���������غ�0.1mol��L-1 NaHCO3��Һ�У�c(Na+)= c(CO32-)+ c(HCO3-)+c(H2CO3)����B��������ʱ��pH=2��CH3COOH��Һ��HCl��Һ�У�ˮ�����������Ϊ10-12mol/L��pH=12�İ�ˮ��NaOH��Һ�У�ˮ�����������Ϊ10-12mol/L,����������Һ����ˮ�����c(H+)��ȣ���C����D. 0.3 mol/L HY��Һ��0��3 mol/L NaOH��Һ�������Ϻ�,�õ�����Һ��NaY��Һ,��֪pH = 9,˵��HY������,����NaY������ˮ��.����Һ�д��������غ㣺c(![]() ) = c(
) = c(![]() ) + c(HY)��Һ��ͬʱ���ڵ���غ㣺c(
) + c(HY)��Һ��ͬʱ���ڵ���غ㣺c(![]() ) + c(
) + c(![]() ) = c(
) = c(![]() ) + c(
) + c(![]() )
)
��1ʽ- 2ʽ�ã�c(![]() ) - c(
) - c(![]() ) = c(
) = c(![]() )��ΪpH= 9, c(
)��ΪpH= 9, c(![]() ) = 10-9mol/L����c(H+) +c(HY)= 1��10-5 mol��L-1����D��ȷ���𰸣�D��
) = 10-9mol/L����c(H+) +c(HY)= 1��10-5 mol��L-1����D��ȷ���𰸣�D��

 �Ķ��쳵ϵ�д�
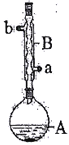
�Ķ��쳵ϵ�д�����Ŀ������ͼ��ʾʵ��װ���У���ʢ����Һ�ٺ���Һ�ڵ��Թ��У�ͨ����������X��������Һ�١���Һ�ھ��������ɵ�����

ѡ�� | ����X | ��Һ�� | ��Һ�� |
A | SO2 | Ca(OH)2 | BaCl2 |
B | Cl2 | AgNO3 | Na2S |
C | NH3 | AgNO3 | AlCl3 |
D | HCl | Na2SiO3 | NaAlO2 |
A. A B. B C. C D. D