��Ŀ����
��1��д��������������ˮ�ĵ��뷽��ʽ�ٱ���������ˮ��______����̼����������ˮ��______��
��2����25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��______��
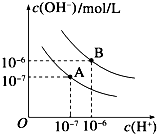
��3����֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
����25��ʱˮ�ĵ���ƽ������ӦΪ______���A����B��������˵������______��
��95��ʱ��0.1mol?L-1��������Һ�У���ˮ��������������ӵ�Ũ����______��
������B��Ӧ�¶��£�pH=2��ijHA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5����HA���ڣ�______���ǿ�ᡱ�������ᡱ����ȷ��������
��2����25�桢101kPa�£�1g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��______��
��3����֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
����25��ʱˮ�ĵ���ƽ������ӦΪ______���A����B��������˵������______��
��95��ʱ��0.1mol?L-1��������Һ�У���ˮ��������������ӵ�Ũ����______��
������B��Ӧ�¶��£�pH=2��ijHA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5����HA���ڣ�______���ǿ�ᡱ�������ᡱ����ȷ��������

��1���ٴ�������������ʣ����ڵ���ƽ�⣬���뷽��ʽΪ��CH3COOH?CH3COO-+H+���ʴ�Ϊ��CH3COOH?CH3COO-+H+��
��̼����������ǿ����ʣ����뷽��ʽΪ��NaHCO3=Na++HCO3-���ʴ�Ϊ��NaHCO3=Na++HCO3-��
��2����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ��32g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ��32=725.76kJ��1mol�״�����Ϊ32�ˣ�������ȫȼ��1mol�״����ɶ�����̼��Һ̬ˮ����725.76KJ���״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+
O2��g��=CO2��g��+2H2O��l����H=-725.76 kJ?mol-1��
�ʴ�Ϊ��CH3OH��l��+
O2��g��=CO2��g��+2H2O��l����H=-725.76kJ?mol-1��
��3����25��c��H+��=c��OH-��=1��10-7��A���ϣ�ˮ�ĵ��������ȹ��̣������¶ȣ�ʹˮ�ĵ���̶������¶�����ʱ���ٽ�ˮ�ĵ��룬ˮ�����ӻ�����ˮ�������ӡ�����������Ũ�ȶ�����ˮ��pH��С������Һ��Ȼ�����ԣ�
�ʴ�Ϊ��A��ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ������̶�С��c��H+����c��OH-��С��
��95��ʱ��0.1mol?L-1��������Һ�У�������Ũ��Ϊ��0.2mol/L��������Һ��ˮ�����������Ũ�ȵ�����Һ�����������ӵ�Ũ�ȣ�
���¶���ˮ�����ӻ�Ϊ��c��H+����c��OH-��=1��10-6��1��10-6=1��10-12����Һ�����������ӵ�Ũ��Ϊ��
=5��10-12mol?L-1��
�ʴ�Ϊ��5��10-12mol?L-1��
������B��Ӧ�¶��£�ˮ�����ӻ�Ϊ1��10-12��pH=2��ijHA��Һ��������Ũ��Ϊ0.01mol/L��pH=10��NaOH��Һ�����������ӵ�Ũ��Ϊ0.01mol/L��������Һ��������Ũ��������������Ũ����ȣ��������Ϻ����Һ��pH=5��˵��HA������ԭpH=2��ijHA��Һ��������Ũ��Ϊ0.01mol/L����HA���ֵ���ģ�����HA�������ᣬ
�ʴ�Ϊ�����ᣮ
��̼����������ǿ����ʣ����뷽��ʽΪ��NaHCO3=Na++HCO3-���ʴ�Ϊ��NaHCO3=Na++HCO3-��
��2����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ��32g�״�ȼ������CO2��Һ̬ˮʱ����22.68kJ��32=725.76kJ��1mol�״�����Ϊ32�ˣ�������ȫȼ��1mol�״����ɶ�����̼��Һ̬ˮ����725.76KJ���״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+
| 3 |
| 2 |
�ʴ�Ϊ��CH3OH��l��+
| 3 |
| 2 |
��3����25��c��H+��=c��OH-��=1��10-7��A���ϣ�ˮ�ĵ��������ȹ��̣������¶ȣ�ʹˮ�ĵ���̶������¶�����ʱ���ٽ�ˮ�ĵ��룬ˮ�����ӻ�����ˮ�������ӡ�����������Ũ�ȶ�����ˮ��pH��С������Һ��Ȼ�����ԣ�
�ʴ�Ϊ��A��ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ������̶�С��c��H+����c��OH-��С��
��95��ʱ��0.1mol?L-1��������Һ�У�������Ũ��Ϊ��0.2mol/L��������Һ��ˮ�����������Ũ�ȵ�����Һ�����������ӵ�Ũ�ȣ�
���¶���ˮ�����ӻ�Ϊ��c��H+����c��OH-��=1��10-6��1��10-6=1��10-12����Һ�����������ӵ�Ũ��Ϊ��
| 1��10-12 |
| 0.2 |
�ʴ�Ϊ��5��10-12mol?L-1��
������B��Ӧ�¶��£�ˮ�����ӻ�Ϊ1��10-12��pH=2��ijHA��Һ��������Ũ��Ϊ0.01mol/L��pH=10��NaOH��Һ�����������ӵ�Ũ��Ϊ0.01mol/L��������Һ��������Ũ��������������Ũ����ȣ��������Ϻ����Һ��pH=5��˵��HA������ԭpH=2��ijHA��Һ��������Ũ��Ϊ0.01mol/L����HA���ֵ���ģ�����HA�������ᣬ
�ʴ�Ϊ�����ᣮ

��ϰ��ϵ�д�
 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ