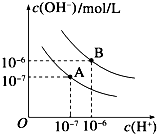
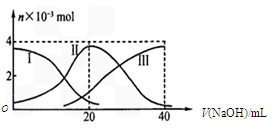
��Ŀ����
���бȽ��У���ȷ����
| A�����µ����ʵ���Ũ����Һ�У�HF��HCN���룬��NaF��Һ��pH��NaCN��Һ�� |
| B��0.2 mol/L NH4Cl��Һ��0.1 mol/LNaOH��Һ�������Ϻ�c(NH4+)��c(Cl��)��c(Na+)��c(OH��)��c(H+) |
| C�����ʵ���Ũ����ȵ�H2S��NaHS�����Һ�У�c(Na+)= c(S2��)+ c(HS��)+ c(H2S) |
| D��ͬŨ�ȵ�������Һ�У���NH4Al(SO4) 2��NH4Cl��CH3COONH4��NH3��H2O��c(NH4+)�ɴ�С��˳���Ǣ٣��ڣ��ۣ��� |
D
���������A�����µ����ʵ���Ũ����Һ�У�HF��HCN���룬HF����ǿǿ��F-ˮ��̶�С������µ����ʵ���Ũ����Һ�У�NaF��Һ��pH��NaCN��ҺС��˵������ȷ��B��0.2 mol/L NH4Cl��Һ��0.1 mol/LNaOH��Һ�������Ϻ���Һ�к���Ũ�ȵ�NaCl��NH4Cl��NH3��H2O����c(Cl��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)������˳�����C�����ʵ���Ũ����ȵ�H2S��NaHS�����Һ�У���S�����ʵ�����Na��2��������2c(Na+)= c(S2��)+ c(HS��)+ c(H2S)��˵������D��ͬŨ�ȵ�������Һ�У���NH4Al(SO4)2��NH4+��Al3+�����ˮ�⣬��NH4Cl(�ο���)��CH3COONH4��NH4+��CH3COO-��ٽ�ˮ���NH3��H2OΪ������ʣ����ֵ��룬��c(NH4+)�ɴ�С��˳���Ǣ٣��ڣ��ۣ�����ȷ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ