��Ŀ����
��A��B��C��D��E��F����ǰ������Ԫ�أ�ԭ������A��B��C��D��E��F��Aԭ��ֻ��һ�����Ӳ���ֻ��1�����ӣ�B��C��Ԫ�صĻ�̬ԭ�Ӿ�����ͬ�ܼ�����I1��B����I1��C�������л�̬Bԭ�ӵ�2p�������3��δ�ɶԵ��ӣ�Dԭ��s��������p��������4��Eԭ�ӵ�3p����ϵõ�1�����Ӻ����������������ӣ�FΪ���ڱ�ǰ�������е縺����С��Ԫ�أ�
��1��д������Ԫ�ص�Ԫ�ط��ţ�C______F______
��2��д��EԪ��ԭ�ӵļ۵��ӹ����ʾʽ______��
��3��B��C��Ԫ��ԭ�ӵĵ縺�Դ�С��B______C���������������
��4��A2C��A2D���۷е㣺A2C______A2D���������������
��5��A��C��Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1��ϳɵĻ����ﻯѧʽΪ______��
��6��BA3������ԭ���ӻ���ʽΪ______�ӻ����û�����Ŀռ乹��Ϊ______��
��1��д������Ԫ�ص�Ԫ�ط��ţ�C______F______
��2��д��EԪ��ԭ�ӵļ۵��ӹ����ʾʽ______��
��3��B��C��Ԫ��ԭ�ӵĵ縺�Դ�С��B______C���������������
��4��A2C��A2D���۷е㣺A2C______A2D���������������
��5��A��C��Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1��ϳɵĻ����ﻯѧʽΪ______��
��6��BA3������ԭ���ӻ���ʽΪ______�ӻ����û�����Ŀռ乹��Ϊ______��
A��B��C��D��E��F����ǰ������Ԫ�أ�ԭ������A��B��C��D��E��F��Aԭ��ֻ��һ�����Ӳ���ֻ��1�����ӣ���AΪ��Ԫ�أ�B��C��Ԫ�صĻ�̬ԭ�Ӿ�����ͬ�ܼ������ߴ��ڴ���ͬ���ڣ���̬Bԭ�ӵ�2p�������3��δ�ɶԵ��ӣ���BΪ��Ԫ�أ���I1��B����I1��C����C��ԭ����������B����CΪ��Ԫ�أ�Eԭ�ӵ�3p����ϵõ�1�����Ӻ����������������ӣ���3p����7�����ӣ���EΪClԪ�أ�FΪ���ڱ�ǰ�������е縺����С��Ԫ�أ���FΪKԪ�أ�Dԭ��s��������p��������4��D�����ܴ��ڵڶ����ڣ���Ϊp�������Ϊ6�����������⣬��ԭ��������ϵ��֪D���ڵ������ڣ���������Ų�Ϊ1s22s22p63s23p4��ΪSԪ�أ�
��1��������������֪��CΪOԪ�أ�FΪKԪ�أ��ʴ�Ϊ��O��K��
��2��EΪClԪ�أ�ԭ�ӵļ۵��ӹ����ʾʽΪ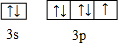 ���ʴ�Ϊ��
���ʴ�Ϊ��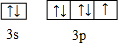 ��
��
��3��ͬ����������ҵ縺�����ʵ縺�Դ�С��N��O���ʴ�Ϊ������
��4��H2O����֮����������������ΪҺ̬��H2S������Ϊ��̬�����۷е㣺H2O��H2S���ʴ�Ϊ������
��5��H��O��Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1��ϳɵĻ����ﻯѧʽΪH2O2���ʴ�Ϊ��H2O2��
��6��NH3������Nԭ�Ӽ۲���Ӷ���Ϊ3+1=4������1�Թµ��Ӷԣ���ȡsp3�ӻ���ʽ���ռ乹��Ϊ�����ͣ��ʴ�Ϊ��sp3�������ͣ�
��1��������������֪��CΪOԪ�أ�FΪKԪ�أ��ʴ�Ϊ��O��K��
��2��EΪClԪ�أ�ԭ�ӵļ۵��ӹ����ʾʽΪ
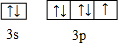 ���ʴ�Ϊ��
���ʴ�Ϊ��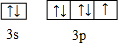 ��
����3��ͬ����������ҵ縺�����ʵ縺�Դ�С��N��O���ʴ�Ϊ������
��4��H2O����֮����������������ΪҺ̬��H2S������Ϊ��̬�����۷е㣺H2O��H2S���ʴ�Ϊ������
��5��H��O��Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1��ϳɵĻ����ﻯѧʽΪH2O2���ʴ�Ϊ��H2O2��
��6��NH3������Nԭ�Ӽ۲���Ӷ���Ϊ3+1=4������1�Թµ��Ӷԣ���ȡsp3�ӻ���ʽ���ռ乹��Ϊ�����ͣ��ʴ�Ϊ��sp3�������ͣ�

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ

