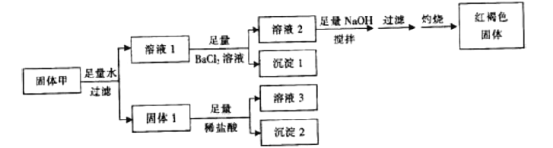
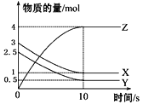
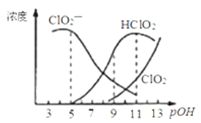
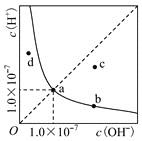
��Ŀ����
����Ŀ�������������Ҫ����оƬ������ϴ��ʴ�̡��ش��������⣺
(1)�����ɱ����ھ��ķ���ϩ�����У����ķ���ϩ�Ľṹ��ʽΪ_____________________��
(2)��ҵ������������������ʱ���辭������(AsF3)��������ϴ�ӵȲ��衣
�ٳ�ȥAsF3�ķ�ӦΪ4AsF3��4KMnO4��4MnO4��2As2O5��4KF��3O2�����÷�Ӧ����������Ϊ___________��
��CoF3����H2O��Ӧ����HF���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________(CoF3��ԭΪCoF2)
������ˮ����������ʱ��������װ���в��뺬��F2��NF3��OF2�е�һ�ֻ���ֵĺ������壬���������ʡ�NF3�ĵ���ʽΪ_________��OF2����Ԫ�صĻ��ϼ�Ϊ___________��OF2����F2��ϡNaOH��Һ��Ӧ��ȡ���÷�Ӧ�����ӷ���ʽΪ____________________________��
���𰸡�![]() As2O5��O2 4CoF3��2H2O=4HF����4CoF2��O2��
As2O5��O2 4CoF3��2H2O=4HF����4CoF2��O2�� 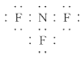 +2 2F2��2OH��=OF2����2F����H2O
+2 2F2��2OH��=OF2����2F����H2O
��������
(1)���ķ���ϩ�ĵ���ΪCF2=CF2��CF2=CF2�����Ӿ۷�Ӧ�õ���
(2)�ٸ��ݻ�ԭ������������Ӧ���õ����������������ԭ�����ϼ����ߣ��ݴ˷�����
�ڸ���������ԭ��Ӧ�Ĺ��ɽ��з�����
��NF3�ĵ���ʽ����NH3��F�ķǽ�����ǿ��O������������ԭ��Ӧ������д��Ӧ����ʽ���ݴ˷�����
(1)���ķ���ϩ�ĵ�����CF2=CF2��CF2=CF2ͨ���Ӿ۷�Ӧ�õ����ķ���ϩ�����ķ���ϩ�Ľṹ��ʽΪ![]() ��
��
�𰸣�![]() ��
��
(2)�����������ǻ�ԭ���������õ�����ԭ���ڷ�Ӧ�л��ϼ����ߣ����ݷ�Ӧ����ʽ��As�Ļ��ϼ��ɣ�3����5�ۣ����ϼ����ߣ�As2O5Ϊ�������O���ֻ��ϼ��ɣ�2�ۡ�0�ۣ����ϼ����ߣ�O2Ҳ���������
�𰸣�As2O5��O2��
��CoF3��ԭ��CoF2��Co�Ļ��ϼ۽��ͣ�Ӧ��Ԫ�صĻ��ϼ����ߣ�O2���Ļ�ԭ��ǿ��F������CoF3��H2O��O������O2����˷�Ӧ����ʽΪ4CoF3��2H2O=4HF����4CoF2��O2����
�𰸣�4CoF3��2H2O=4HF����4CoF2��O2����
��NF3���ڹ��ۻ���������ʽΪ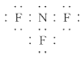 ��F�ķǽ�����ǿ��O��F�Ļ��ϼ�Ϊ��1�ۣ���O�Ļ��ϼ�Ϊ��2�ۣ�OF2����F2��NaOH��Ӧ��F2��������������2��O������OF2����������ԭ��F���������ӷ�Ӧ����ʽΪ2F2��2OH��=OF2����2F����H2O��
��F�ķǽ�����ǿ��O��F�Ļ��ϼ�Ϊ��1�ۣ���O�Ļ��ϼ�Ϊ��2�ۣ�OF2����F2��NaOH��Ӧ��F2��������������2��O������OF2����������ԭ��F���������ӷ�Ӧ����ʽΪ2F2��2OH��=OF2����2F����H2O��
�𰸣�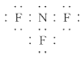 ��+2��2F2��2OH��=OF2����2F����H2O��
��+2��2F2��2OH��=OF2����2F����H2O��