��Ŀ����
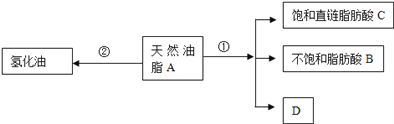
����Ŀ����ϩ�ͱ����л��ϳɵĻ���ԭ�ϣ�ͼ�����л���H�� ���ĺϳ�·�ߣ����ֲ����������ʡ�ԣ���
���ĺϳ�·�ߣ����ֲ����������ʡ�ԣ���

��֪��![]() ��
��
��ش��������⣺
��1��B�Ĺ���������Ϊ__________________����Ӧ�٢ڵķ�Ӧ���ͷֱ�Ϊ_________��_____________��E������Ϊ_______________��
��2��д����Ӧ�۵Ļ�ѧ����ʽ��________________��
��3��P����ϩ�����ʽ��ͬ������Է�������Ϊ56��P�����п��ܵĽṹ����P����ĿΪ__________�֣������������칹����д��P�����ڻ�����ͬ���칹��Ľṹ��ʽ��____________________��
��4��![]() �DZ���ͬϵ�M�ĺ˴Ź���������2��壬�ҷ������Ϊ2��3��M�Ķ��ȴ�����________�֡�
�DZ���ͬϵ�M�ĺ˴Ź���������2��壬�ҷ������Ϊ2��3��M�Ķ��ȴ�����________�֡�
��5����֪�� �������������̣������FΪԭ���Ʊ�G�ĺϳ�·�ߣ�
�������������̣������FΪԭ���Ʊ�G�ĺϳ�·�ߣ�
 ________________________�����Լ���ѡ����
________________________�����Լ���ѡ����
���𰸡�ȩ�� ������Ӧ ȡ����Ӧ �ױ� 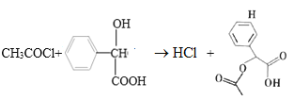 4
4 ![]() 7
7 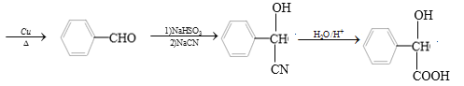
��������
��ϩ��ˮ�ӳ�����A�Ҵ����Ҵ�����������������B��ȩ��������C���ᣬ����֪��Ϣ֪�������SOCl2����D��CH3COCl����ȡ������E�ױ�������ȡ������1-�ȼױ���F�������ɷ�Ӧ�۵��ƻ�GΪ��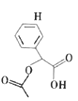 ��
��
��1��B����ȩ��B�Ĺ���������Ϊȩ������Ӧ���Ǵ�����Ϊȩ��������������Ӧ����Ӧ���DZ���Ϊ�ױ�����ȡ����Ӧ��E�Ǽױ���
�ʴ�Ϊ��ȩ����������Ӧ��ȡ����Ӧ���ױ���
��2����Ӧ����D��E����H�ķ�Ӧ����ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3����ϩ�����ʽ��ͬΪ![]() ��n=1������Ϊ14���� P����Է�������Ϊ56����n=4�������Ʋ�P�Ļ�ѧʽΪC4H8�����ܵĽṹΪ��
��n=1������Ϊ14���� P����Է�������Ϊ56����n=4�������Ʋ�P�Ļ�ѧʽΪC4H8�����ܵĽṹΪ��![]() ��
��![]() ��
��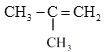 ��
��![]() �������ֽṹ ������P�Ļ�����ͬ���칹��Ľṹ��ʽΪ��
�������ֽṹ ������P�Ļ�����ͬ���칹��Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��4��![]() ��
��
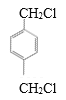
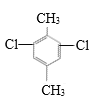
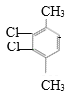
��4��![]() �DZ���ͬϵ�M�ĺ˴Ź���������2��壬�ҷ������Ϊ2��3����M�DZ��������������Ļ������ ֻ������壬˵�������ʸ߶ȶԳƣ�������λ�ڶ�λ����M�ǶԶ��ױ���M�Ķ��ȴ��������������
�DZ���ͬϵ�M�ĺ˴Ź���������2��壬�ҷ������Ϊ2��3����M�DZ��������������Ļ������ ֻ������壬˵�������ʸ߶ȶԳƣ�������λ�ڶ�λ����M�ǶԶ��ױ���M�Ķ��ȴ�������������� ��
�� ��
�� ��
�� ��
�� ��
��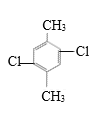 ��
�� ����7�֣�
����7�֣�
�ʴ�Ϊ��7��
��5�������F��![]() ��Ϊԭ���Ʊ�G��
��Ϊԭ���Ʊ�G��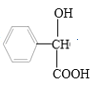 ���ĺϳ�·�ߣ���֪��
���ĺϳ�·�ߣ���֪�� ��
��![]() �����кϳ�·�ߣ�
�����кϳ�·�ߣ�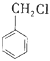

 ��
��
�ʴ�Ϊ ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�