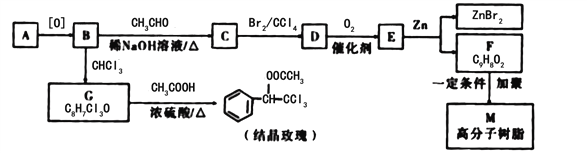
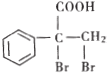
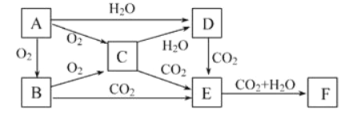
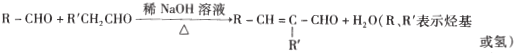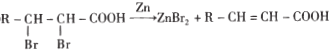��Ŀ����
����Ŀ�����º����£���2molA�����2molB����ͨ�����Ϊ2L���ܱ������з������·�Ӧ��2A(g)+B(g)![]() xC(g)+2D(s)��2minʱ��Ӧ�ﵽƽ��״̬����ʱʣ��1.2molB�������C��Ũ��Ϊ1.2mo1��L��1��
xC(g)+2D(s)��2minʱ��Ӧ�ﵽƽ��״̬����ʱʣ��1.2molB�������C��Ũ��Ϊ1.2mo1��L��1��
(1)�ӿ�ʼ��Ӧ���ﵽƽ��״̬������C��ƽ����Ӧ����Ϊ____________________��
(2)x =________��
(3)A��ת������B��ת����֮��Ϊ___________��
(4)���и������Ϊ�÷�Ӧ�ﵽƽ��״̬�ı�־����_________��
A��ѹǿ���ٱ仯
B�������ܶȲ��ٱ仯
C������ƽ����Է����������ٱ仯
D��A������������B����������֮��Ϊ2��1
(5)��ʹ��Ӧ�ﵽƽ��ʱ��C�����ʵ�������������ƽ����ȣ���ʼ����A��B�����ʵ���n(A)��n(B)֮��Ӧ����Ĺ�ϵΪ ________________________��
���𰸡� 0.6mo1��L��1��min��1 3 2:1 BC n(A) = n(B)��n(A):n(B)=1:1
����������1��2min�ﵽƽ�⣬C��Ũ��Ϊ1.2mol/L���������C��ƽ����Ӧ����Ϊ1.2mol/L��2min=0.6mol/��L��min����
��2�� 2A(g)+B(g)![]() xC(g)+2D(s)
xC(g)+2D(s)
��ʼ����mol�� 2 2 0
ת������mol�� 1.6 0.8 0.8x
ƽ������mol�� 0.4 1.2 0.8x
��0.8xmol��2L��1.2mol/L�����x��3
��3��A��B��ת����֮��Ϊ![]() ��
��
��4��A���÷�Ӧ��ѹǿʼ�ղ��䣬�����ж�ƽ�⣬A����B��������������ڱ仯���������ܶȲ��ٱ仯���ﵽƽ�⣬B��ȷ��C��������������ڱ仯������������ʵ������䣬������ƽ����Է����������ٱ仯���ﵽƽ�⣬C��ȷ��D��A������������B����������֮��Ϊ2��1����ϵʼ�մ��ڣ������ж�ƽ�⣬D����ѡBC��
��5����ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�������������ƽ����ȣ���ԭƽ��Ϊ��Чƽ�⣬����x=3����Ӧǰ������Ļ�ѧ���������䣬����ʼ�����A��B���ʵ���n��A����n��B��Ӧ��ԭƽ�����Ϊ1��1����n��A��=n��B����