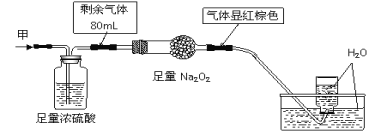
��Ŀ����
����Ŀ�������������г��õ�ȥ����ϴ�Ӽ���ijͬѧ����̼���ƾ��壨Na2CO310H2O������100mL1molL-1�Ĵ�����Һ����ش��������⣺
��1����������ҪNa2CO310H2O������Ϊ________g��
��2��ȡ����Һ20mLϡ�͵�100mL�����Һ��c��Na+��Ϊ_______molL-1��
��3���������ʵ�飬����ͼ��ʾ�������⣬����Ҫ���ӵIJ���������__________________________��
��4��������Һʱ������ʵ�������ʹ������ҺŨ��ƫ�͵���__________��
A������ƿ����ˮ��δ�������ﴦ��
B�����ݲ���ʱ�����ӿ̶���
C��������ƿ��ת��ʱ������Һ�彦��
D�����ݺ�ת����ƿ���Σ�����Һ����͵���ڿ̶��ߣ��ٲ��Ӽ���ˮ���̶���
���𰸡�28.6 0.4 �������ͽ�ͷ�ι� CD
��������
��1������m=cVM����Na2CO3��10H2O��������
��2������c=n/V���㣻
��3������ʵ������IJ����Լ�ÿ������ȷ������������
��4������c=n/V�������������ʵ����ʵ��������Һ�����Ӱ���жϡ�
��1��ʵ��������100mL1mol��L��1Na2CO3��Һ��ҪNa2CO3�����ʵ���Ϊ��0.1L��1mol��L��1=0.1mol��Na2CO3��10H2O�����ʵ���Ϊ0.1mol��Na2CO3��10H2O������Ϊ��0.1mol��286g��mol��1=28.6g��
��2��ȡ����Һ20mLϡ�͵�100mL�����Һ��c��Na+��=0.02L��1mol��L��1��2��0.1L=0.4molL-1��
��3�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ��ʴ�Ϊ������������ͷ�ιܣ�
��4�� A������ƿ����ˮ��δ�������ﴦ���������ʺ��ܼ�����Ӱ�죬����������Һ�����ʵ���Ũ�Ȳ��䣬��A��ѡ��
B�����ݲ���ʱ�����ӿ̶��ߣ�����ˮ�����㣬�����ҺŨ��ƫ�ߣ���B��ѡ��
C��������ƿ��ת��ʱ������Һ�彦���������������ʧ��ʹ������ҺŨ��ƫ�ͣ���Cѡ��
D�����ݺ�ת����ƿ���Σ�����Һ����͵���ڿ̶��ߣ��ٲ��Ӽ���ˮ���̶��ߣ���Һ���ƫ��������Һ��Ũ��ƫ�ͣ���Dѡ��
��ѡCD��