��Ŀ����
6�� 50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����з��̵������ɼ����к��ȣ��ش��������⣺
50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����з��̵������ɼ����к��ȣ��ش��������⣺��1����ʵ��װ���Ͽ���ͼ��ȱ�ٵ�һ�ֲ�����Ʒ�ǻ��β����������
��2���ձ���������ֽ���������DZ��£���������ɢʧ��
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵƫС���ƫ����ƫС������Ӱ�족��
��4��ʵ���и���60mL 0.50mol/L�����60mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ������ȡ�����ȡ����������к�����ȣ������ȡ�����ȡ���������������Ϊ�к�����ָ�������кͷ�Ӧ����1molH2O���ų��������������������أ�
��5��ʵ���и���8.0mL 15mol/LŨ������Һ��60mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ�飨4����ȣ������к���ƫС���ƫ����ƫС������Ӱ�족���������ɣ�Ũ����ϡ��ʱ�ų�������
��6��ʵ���и���60mL0.50mol/L�����60mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ�飨4����ȣ������к���ƫ���ƫ����ƫС������Ӱ�족���������ɣ�������������ʣ��������ȣ�
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2��ʵ��ɰܵĹؼ��DZ��¹������ݴ�ȷ���ձ���������ֽ�������ã�
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5��Ũ����ϡ��ʱ�ų�������
��6��������������ʣ��������ȣ�
��� �⣺��1���������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������ʴ�Ϊ�����β����������
��2��ʵ��ɰܵĹؼ��DZ��¹������ձ���������ֽ�������ñ��£���������ɢʧ���ʴ�Ϊ�����£���������ɢʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫС��
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������60mL 0.50mol/L�����60mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������60mL 0.50mol/L�����60mL 0.55mol/L NaOH��Һ���з�Ӧ������к�����ֵ��ȣ��ʴ�Ϊ������ȣ���ȣ���Ϊ�к�����ָ�������кͷ�Ӧ����1molH2O���ų��������������������أ�
��5��Ũ����ϡ��ʱ�ų�����������8.0mL 15mol/LŨ������Һ��60mL 0.55mol/L NaOH��Һ���з�Ӧ���ų�����ƫ�࣬�к��ȵ���ֵƫ���к���ƫС��
�ʴ�Ϊ��ƫС��Ũ����ϡ��ʱ�ų�������
��6��������������ʣ��������Ϊ���ȹ��̣����Ը���60mL0.50mol/L�����60mL 0.55mol/L NaOH��Һ���з�Ӧ����Ӧ�ų�������ƫС���к��ȵ���ֵƫС�����к���ƫ��
�ʴ�Ϊ��ƫ������������ʣ��������ȣ�
���� ���⿼�����к��ȵIJⶨ��������Ŀ�ѶȲ���ע�����ղⶨ�к��ȵ���ȷ��������ȷʵ����������йؼ����ھ����ܼ�������ɢʧ��ʹ�ⶨ�������ȷ��

��1��O2-�����ӽṹʾ��ͼΪ
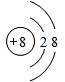 ��CS2�ľ�������Ϊ���� ���壻
��CS2�ľ�������Ϊ���� ���壻��2��O��Cl��Ԫ���γɵĵ��ʺͻ����ﳣ����ɱ���������Ծ���ClO2��O3��Cl2��д��ѧʽ����д���֣���
��3��CH3OH�ڳ�����ΪҺ̬���е�����������Ҫԭ���Ǽ״�����֮�����γ���������鲻�ܣ�
��4��Cl2��һ�ִ�����Ⱦ�Һ�ȴ��������е�˵�������£����֣���
| ���� |  |
| ����Ҫ�� | Զ�������ĩ���������ࡢ�������ʣ�������������� |
| й©���� | NaOH��NaHSO3��Һ���� |
| ��װ | ��ƿ |
����Һ��й©�����������ڸ�ƿ�������뱽�ķ�Ӧ���Լӿ죬ԭ����Fe��FeCl3���ܴ����������ķ�Ӧ��
�۽�Cl2ͨ������KOH��Һ�У������п�����KCl��KClO��KClO3������Һ��c��Cl-����c��ClO-��=11��1ʱ����c��ClO-����c��ClO3-����ֵ����1��2
��5��þ��һ�ֽϻ��õĽ�����Mg��Ca���ƣ�Ҳ����C�γ�ij����ˮ������ӻ������֪�û�����0.1mol��ˮ��ȫ��Ӧ����0.1mol��ij�����壮�����屻��ˮȫ�����պ���ˮ����2.8g����д����ˮ�ⷴӦ����ʽMgC+2H2O=Mg��OH��2+C2H4��
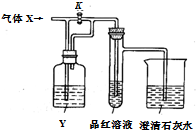 ��ͼ�Ǽ����������ʵ�ʵ��װ�ã���װ���л���ͨ������X�����رջ���K��Ʒ����Һ�ޱ仯������ʯ��ˮ����ǣ�������K��Ʒ����Һ��ɫ���ݴ��жϣ�����X��Һ��Y�����ǣ�������
��ͼ�Ǽ����������ʵ�ʵ��װ�ã���װ���л���ͨ������X�����رջ���K��Ʒ����Һ�ޱ仯������ʯ��ˮ����ǣ�������K��Ʒ����Һ��ɫ���ݴ��жϣ�����X��Һ��Y�����ǣ�������| ѡ�� ���� | A | B | C | D |
| X | H2S | SO2 | CO2 | Cl2 |
| Y | Ũ���� | NaHCO3������Һ | Na2SO3 ��Һ | NaHSO3 ��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | CH3-C��CH3��=CH-CH3 | B�� | CH3-CCl=CH-CH3 | C�� | 1-��ϩ | D�� | ��ϩ |

�������жϴ�����ǣ�������
| A�� | B�к��еĹ�����Ϊ�ǻ� | B�� | ����̼������Һ����B��D | ||
| C�� | D�к���C�TO����E������Ϊ�������� | D�� | B+D��E��Ӧ��װ����ͼ |
| A�� | ú | B�� | ʯ�� | C�� | ��Ȼ�� | D�� | �������� |
| A�� | 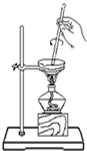 �ӵ�ˮ�з������ | B�� |  ������Һ��NH4+�Ĵ��� | ||
| C�� | 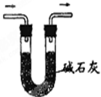 ����SO2���� | D�� |  �ռ�HCl���� |
| A�� | ������������ϩʹ�������������Һ��ɫ�ķ�Ӧ������ȡ����Ӧ | |
| B�� | �����ʡ����ۡ���֬�ȶ�����������ˮ�Ⲣ�ṩ���� | |
| C�� | ��ϩ��������������е�����ԭ�Ӷ���ͬһƽ���� | |
| D�� | ʯ���ѽ����ҪĿ����������͵������͵IJ�����������ʯ�ʹ��ѻ�����ҪĿ���ǵõ��������ϩ����ϩ����̬������ |
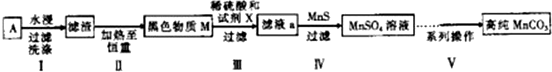
��1������п�̸ɵ�صĸ���������Zn���ѧʽ����
��2���ڢ�����Ŀ���dz�ȥ̼�ۣ�
��3���ڢ��������Ƕ���Һa������ȳ��ӣ���ȥZn2+�����ӷ���ʽΪZn2++MnS=ZnS+Mn2+������֪��Ksp��MnS��=2.5��10-13��Ksp��ZnS��=1.6��10-24��
��4��Ϊѡ���Լ�X������ͬ�����£��ֱ���5g ��ɫ����M�����Ʊ�MnSO3��ʵ�飬�õ��������±���
| ʵ���� | �Լ�X | MnSO4/g |
| 1 | � | 2.3595 |
| 2 | ���� | 2.7184 |
| 3 | ������ | 2.9911 |
| 4 | 30%�������� | 3.7349 |
�ڵڢ�����ѡ��������Լ�X��M����Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽΪMnO2+H2O2+H2SO4=MnSO4+2H2O+O2����
��5����֪��MaCO3������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻Mn��OH��2��ʼ����ʱpHΪ7���벹��������²�����
�ڢ���ϵ�в����ɰ�һ�����̽��У��벹����ɲ��������ڢ���ϵ�в����пɹ�ѡ�õ��Լ���NaHCO3���Ҵ���

����1������NaHCO3������pH��7.7��
����2�����ˣ�������ˮϴ��2��3��
����3�������Һ������SO42-�ѳ��ɾ���
����4����������ˮ�Ҵ�ϴ��2��3�Σ�
����5�����º�ɣ�
��6������1���ܷ�����Ӧ�����ӷ���ʽMn2++2HCO3-=MnCO3��+H2O+CO2����