��Ŀ����
������ͼʵ���������ý��۲���ȷ���ǣ�������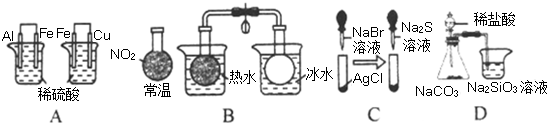
|
������A��ԭ����У���Ϊ���õĽ�����������
B����������Ϊ����ɫ���壬������������ɫ��������ɫ�仯��֪ƽ�ⷢ���ƶ���
C���ܶȻ�ԽС�����ʵ��ܽ���ԽС��
D���ȽϷǽ����ԣ�Ӧ��Ԫ�ض�Ӧ������������ˮ���
B����������Ϊ����ɫ���壬������������ɫ��������ɫ�仯��֪ƽ�ⷢ���ƶ���
C���ܶȻ�ԽС�����ʵ��ܽ���ԽС��
D���ȽϷǽ����ԣ�Ӧ��Ԫ�ض�Ӧ������������ˮ���
����⣺A��ԭ����У���Ϊ���õĽ���������������ձ��������������ݣ�˵��Al��Fe���ã��ұ��ձ���ͭ���������ݣ�˵��Fe��Cu���ã���A��ȷ��
B����������Ϊ����ɫ���壬������������ɫ���������������������ķ����ƶ���˵������Ӧ���ȣ���B��ȷ��
C���ܶȻ�ԽС�����ʵ��ܽ���ԽС����ɫ�����ȱ�Ϊ����ɫ�����Ϊ��ɫ����֤���ܶȻ���Ksp����AgCl��AgBr��Ag2S����C��ȷ��
D���ȽϷǽ����ԣ�Ӧ��Ԫ�ض�Ӧ������������ˮ�����D����
��ѡD��
B����������Ϊ����ɫ���壬������������ɫ���������������������ķ����ƶ���˵������Ӧ���ȣ���B��ȷ��
C���ܶȻ�ԽС�����ʵ��ܽ���ԽС����ɫ�����ȱ�Ϊ����ɫ�����Ϊ��ɫ����֤���ܶȻ���Ksp����AgCl��AgBr��Ag2S����C��ȷ��
D���ȽϷǽ����ԣ�Ӧ��Ԫ�ض�Ӧ������������ˮ�����D����
��ѡD��
���������⿼�黯ѧʵ�鷽�������ۣ��漰�����ԡ��ǽ����ԵıȽ��Լ�ԭ���֪ʶ�����ܵ���ʵ��ܽ�ƽ�����⣬�����ڿ���ѧ�����ۺ����û�ѧ֪ʶ��������������������Ŀ�ѶȲ���ע������������ʵ���ͬ�Լ����ʵ�����������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
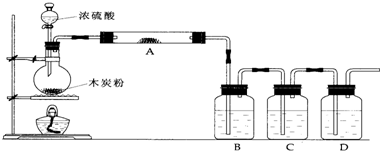
 �о���ѧϰС��Ϊ̽��Cu��ŨH2SO4��Ӧ�������SO2�����ʣ��������ʵ��װ�ã�
�о���ѧϰС��Ϊ̽��Cu��ŨH2SO4��Ӧ�������SO2�����ʣ��������ʵ��װ�ã�
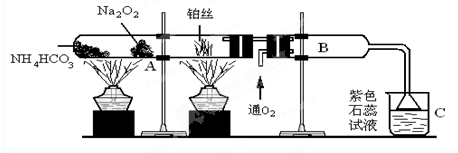
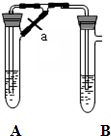 �ס�����ͬѧ����ȡ������Fe��OH��2��������ͼ��ʾ��װ�ý������飮A������Fe��ϡ���ᣬB������NaOH��Һ���ش��������⣮
�ס�����ͬѧ����ȡ������Fe��OH��2��������ͼ��ʾ��װ�ý������飮A������Fe��ϡ���ᣬB������NaOH��Һ���ش��������⣮