��Ŀ����
��8�֣���֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl����ClO����ClO3�����ֺ���Ԫ�ص����ӣ�����ClO����ClO3���������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
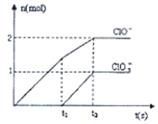
��1��t1ǰ������������________���ѧʽ����
��2��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ�� ��
��3����ʯ�����к���Ca��OH��2�����ʵ����� mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� ��

��1��t1ǰ������������________���ѧʽ����
��2��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ�� ��
��3����ʯ�����к���Ca��OH��2�����ʵ����� mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� ��
| A��NaCl��Cl2 | B��NaCl��NaClO | C��NaClO3��NaClO4 | D��NaCl��NaClO3 |
��1��Ca(ClO)2 ��2��5Ca(OH)2+5Cl2=5Ca2++7Cl-+2ClO-+ClO3��+5H2O ��3��5 ��4��D
��1������ͼ���֪��t1֮ǰ����������Ca(ClO)2��
��2��t2ʱ�������������Ca(ClO)2������Ca(ClO3)2�����ߵ����ʵ����ֱ�����1mol��0.5mol������ת�Ƶ�����7mol��������3.5mol�Ȼ��ƣ���˷���ʽΪ5Ca(OH)2+5Cl2=5Ca2++7Cl-+2ClO-+ClO3��+5H2O��
��3������ԭ���غ��֪���������Ƶ����ʵ�����1mol��0.5mol��3.5mol��5mol��
��4���������ƹ��屬ը����������������ԭ��Ӧ��������Ԫ�صĻ��ϼۼ������ߵģ����н��͵ģ����ѡD��
��2��t2ʱ�������������Ca(ClO)2������Ca(ClO3)2�����ߵ����ʵ����ֱ�����1mol��0.5mol������ת�Ƶ�����7mol��������3.5mol�Ȼ��ƣ���˷���ʽΪ5Ca(OH)2+5Cl2=5Ca2++7Cl-+2ClO-+ClO3��+5H2O��
��3������ԭ���غ��֪���������Ƶ����ʵ�����1mol��0.5mol��3.5mol��5mol��
��4���������ƹ��屬ը����������������ԭ��Ӧ��������Ԫ�صĻ��ϼۼ������ߵģ����н��͵ģ����ѡD��

��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ
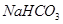 ��
��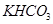 ��ɵĻ����18.400 g,��100 mL���ᷴӦ��(�����漰�����������Ϊ��״����,���ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)
��ɵĻ����18.400 g,��100 mL���ᷴӦ��(�����漰�����������Ϊ��״����,���ʱ�����ô���ĸ��ʽ�ӱ�ʾ��) 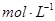 ��
��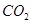 �����Ϊ L��
�����Ϊ L�� �������飩�к���6 NA���ۼ�
�������飩�к���6 NA���ۼ�