��Ŀ����
����Ŀ����NA��ʾ����٤����������ֵ������˵������ȷ������ ��
A. ��״���£�2.24L 1H2��0.4g2H2������0.2NA������
B. ���Ե缫��ⱥ��ʳ��ˮ������·��ͨ��2NA�����ӣ���������������22.4L
C. ���³�ѹ�£�23g NO2��N2O4�Ļ�������к��е�ԭ����Ϊ1.5NA
D. 1mol Na2CO3�����к��е�CO32-��Ŀһ��ΪNA
���𰸡�B
��������A����״���£�2.24L 1H2�����ʵ���Ϊ0.1mol����0.4g2H2�����ʵ���ҲΪ0.1mol����1H2��2H2����������Ϊ2������0.1mol1H2��2H2������0.2NA�����ӣ�ѡ��A��ȷ��B�����Ե缫��ⱥ��ʳ��ˮ�������缫��ӦΪ2Cl- -2e-=Cl2��������·��ͨ��2NA�����Ӽ�2mol����������������1mol������һ���DZ�״�������������һ����22.4L��ѡ��B����C��NO2��N2O4�Ļ������ɿ��������ʽΪNO2������23g NO2��N2O4�Ļ�������к��е�ԭ����=![]() ��3��NA =1.5NA��ѡ��C��ȷ��D��̼�����������Ӻ�̼������ӹ��ɣ�1mol Na2CO3�����к��е�CO32-��Ŀһ��ΪNA��ѡ��D��ȷ����ѡB��
��3��NA =1.5NA��ѡ��C��ȷ��D��̼�����������Ӻ�̼������ӹ��ɣ�1mol Na2CO3�����к��е�CO32-��Ŀһ��ΪNA��ѡ��D��ȷ����ѡB��

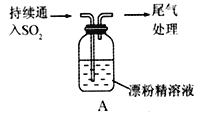
����Ŀ����I��SO2����Ϊ��ɫ���壬��ǿ�Ҵ̼�����ζ��������Ҫ��Ⱦ��֮һ������һ���Ļ�ԭ�ԣ�̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��
��1����Ҫ��FeCl3��Һ����ȡ���壬�ڱ�����е�ʵ����������У�û���õ��IJ���������________________������ĸ����
a.�ƾ��� b.��ƿ c.©�� d.�ձ� e.������
��2��װ��A�е�������__________________��д��B�з�����Ӧ�����ӷ���ʽ��__________________��
��3��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ��ȡA�е���Һ���ֳ����ݣ����������������
�����٣����һ����Һ�м�����������KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ���ڶ�����Һ�м���KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
���������в���������_______________������ţ���
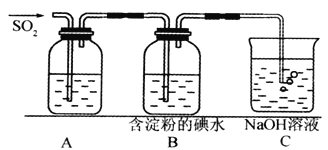
����SO2����ˮ���������ᣬ�����������ǿ�ڴ����ᡣѡ�������װ�ú�ҩƷ��̽��������������������ǿ����
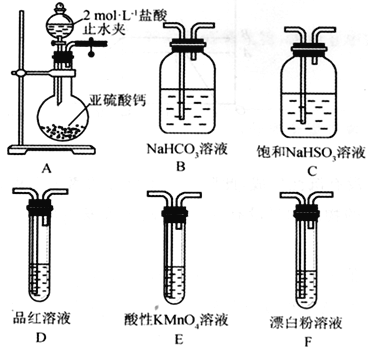
��4��װ����ȷ������˳��ΪA��________________________________��D��F������װ��B��������_________��֤�������������ǿ�ڴ����������Ϊ_____________________________��
����ijͬѧ��SO2��Ư�۾��ķ�Ӧ������ʵ��̽����
���� | ���� |
ȡ4 gƯ�۾����壬����100 mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
| ����Һ���ֻ��ǣ�����Ϊ����ɫ ���Ժ���������ɫ����������ɫ��ȥ |
��5��C12��Ca(OH)2��Ӧ��ȡƯ�۾��Ļ�ѧ����ʽ��______________________________________��
��6��pH��ֽ��ɫ�ı仯˵��Ư�۾���Һ���е�������__________________________________��
��7�����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾�����Ч�ɷ�ClO-��Cl-������Ӧ��д��Ư�۾������������·�����Ӧ�����ӷ���ʽ��_________________________________��