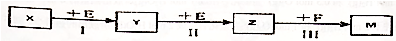��Ŀ����
12����������������Դ���⣬������Դ�Ŀ��������þ��й�����ǰ������1�������йغ�ˮ��Դ�ۺ����õ�˵����ȷ����bd������ţ�
a����ⱥ��ʳ��ˮ���Ƶ���
b���Ӻ�������ȡ���漰��ѧ�仯
c�����ó�ϫ�����ǽ���ѧ��ת��Ϊ����
d�����õ����������ԴӺ�ˮ�л�ȡ��ˮ
��2��ij��ȤС���Ժ�ˮΪԭ��ģ�ҵ������ȡNaCl��Mg��Br2����Ҫ�������£�
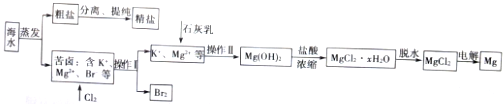
�����������в����õ��IJ����������ձ���©���Ͳ����������������ƣ���
��Ϊ��ȥ�����е�Ca2+��Mg2+��SO42-���ɼ�����Լ��У�a��Na2CO3��Һ b��BaCl2��Һ c��NaOH��Һ�������Լ��ĺ���˳��Ϊbac ��cba ��bca������ţ�
��Mg��OH��2�����еμ����ᣬŨ���������õ�MgCl2��xH2O���壬ȡ60.9g���壬��ˮ����⣨������ģ������յõ�7.2gþ����x����6��
��3������ͼװ��ģ��Ӻ�ˮ�и������ʵ�飮
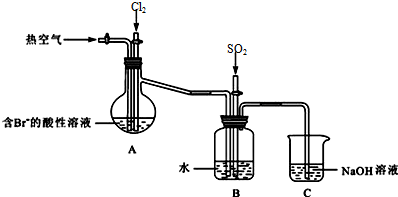
Aװ����ͨ��Cl2һ��ʱ���ͨ�ȿ�������Br2������Bװ�ã�
������a��������������ƿ��
��Bװ���У�Br2��SO2��ԭ���÷�Ӧ�Ļ�ѧ����ʽΪBr2+SO2+2H2O=H2SO4+2HBr��
��Cװ����NaOH��Һ������������β������ֹCl2��SO2��Br2�Ի�����Ⱦ��
���� ��1����ˮ�и���Na��Cl��Mg��Br��I��Ԫ�أ��Ӻ�ˮ�л�ȡ�ơ�þ�������ȵ��ʣ�Ҫ�������Ȳ�����Ϊ��ѧ�仯����ˮ��������ɵõ���ˮ�����������ᾧ�ɵõ��Ȼ��ƣ���ϫ�����ǽ���ϫ��ת��Ϊ���ܣ�������ˮ��Ӧ��ˮ�뺣ˮ�е��ν��з��룬���÷��������������Լ����ӽ��������Դ˽����⣻
��2���ٹ���װ����Ҫ�ձ���©�����������ȣ�
���ȷ���NaCl��Һ�л��е�Ca2+��Mg2+��SO42-��HCO3-���������ص��ѡ�õ��Լ���Ӧ���ȼ����������ƣ�Ȼ������Ȼ������ټ���̼���ƣ���������������ÿ���Լ������������֤���������ʶ��ܳ�ȥ����Ӧ����ü��ȷ�������ȥʣ����Ȼ��⣻
�ۼ���þԪ�����ʵ���������þԪ���غ�����Ȼ�þ����������Ͼ��������õ�ˮ������������ˮ�����ʵ��������ݻ�ѧʽ����x��
��3����װ��A�Ǵ�֧�ܵ���ƿΪ������ƿ��
��ͨ�����������SO2+Br2+2H2O=4H++2Br-+SO42-�������������ã�������������������������壻
��β���к���Cl2��Br2��SO2���ж����壬����NaOH��Һ���գ�
��� �⣺��1��a����ˮ�к����Ȼ��ƣ�������ˮ�����Ƶ��Ȼ��ƣ���������Ȼ��Ƶõ������ƣ����ǻ��õĽ���������Ȼ�����Һ���ܵõ������ƣ���a����
b����ˮ�еĵ�Ԫ���Ե����ӵ���ʽ���ڣ���Ҫ�������������ܻ�õ��ʵ⣬Ϊ��ѧ�仯����b��ȷ��
c����ϫ�����ǽ���ϫ��ת��Ϊ���ܣ���c����
d�����õ���������ʹ��Ӧ������ͨ����Ĥ�ԴﵽӲˮ������Ч�������õ����������ԴӺ�ˮ�л�ȡ��ˮ����d��ȷ��
��ѡbd��
��2����������������Ҫ�ǹ��˲������õ��IJ����������ձ���©���Ͳ�������
�ʴ�Ϊ����������
���ȼ���������c��NaOH����ȥþ���ӣ��� HCO3-ת����̼�����Ȼ��������b��BaCl2����ȥSO42-��CO32-���ټ������c��Na2CO3 ��ȥCa2+�����˺����Լ��ĺ���˳�����Ϊbac ��cba ��bca��
�ʴ�Ϊ��bac ��cba ��bca��
��ȡMg60.9gCl2��xH2O���壬��ˮ����⣨������ģ������յõ�7.2gþ��n��Mg2+��=$\frac{7.2g}{24g/mol}$=0.3mol��m��MgCl2��=0.3mol��95g/mol=28.5g��
������ˮ������=60.9g-28.5g=32.4g
n��H2O��=$\frac{32.4g}{18g/mol}$=1.8mol
n��MgCl2����n��H2O��=1��x=0.3��1.8��
x=6
�ʴ�Ϊ��6��
��3����װ��A�Ǵ�֧�ܵ���ƿΪ������ƿ��
�ʴ�Ϊ��������ƿ��
����ˮ�ܰ�SO2���������廯������ᣬ��Ӧ�Ļ�ѧ����ʽΪ��Br2+SO2+2H2O=H2SO4+2HBr��
�ʴ�Ϊ��Br2+SO2+2H2O=H2SO4+2HBr��
����������������δ��Ӧ���Cl2��Br2��SO2���ж����壬��ֹ��Ⱦ������
�ʴ�Ϊ������β������ֹCl2��SO2��Br2�Ի�����Ⱦ��
���� ���⿼�麣ˮ���ۺ�Ӧ�á����ӷ���ʽ����д��β�����������е��Ѷȵ����⣬����ע�ػ�����������˫�飬����������ѧ������˼ά�����ʹ���˼ά������Ҳ�����ڿ���ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д��ٱ�����ʹ����KMnO4��Һ��ɫ
�ڱ���6��̼̼����ȫ��ͬ
�۱���һ�����û��ͬ���칹��
��ʵ���Ҳ���ڶ��ױ�ֻ��һ�ֽṹ
�ݱ�����ʹ��ˮ��Ӧ����ɫ��
| A�� | �ڢۢܢ� | B�� | �٢ۢܢ� | C�� | �٢ڢۢ� | D�� | �٢ڢܢ� |
| A�� | ú��������Һ�������������仯 | |
| B�� | ͨ��ʯ�ͷ���õ��������Ǵ����� | |
| C�� | ʯ���ѽ���Ϊ�˵õ���ϩ����ϩ����̬������ | |
| D�� | ����IJ�������ȡ�������ױ��ȷ���������Ϊú�к��б��ͼױ� |
| A�� | �ڹ���Ԫ����Ѱ�Ұ뵼����� | |
| B�� | Ԫ�����ڱ��е�һ��Ԫ�ض��Ǽ����Ԫ�� | |
| C�� | ����Ԫ�����ڱ��ǰ���Ԫ�����ԭ��������С�����˳������ | |
| D�� | M�����Ϊ��������������Ԫ�ص����������Ԫ��ԭ�ӵ�M���������� |
| A�� | F-�Ľṹʾ��ͼ�� | B�� | CH4���ӵı���ģ�ͣ� | ||
| C�� | CCl4�ĵ���ʽ�� | D�� | ����Ľṹ��ʽ�� |
| A�� | 1mol/L | B�� | 10mol/L | C�� | 0.01mol/L | D�� | 0.1mol/L |
| A�� |  �� �� | B�� | �����2��2һ�������� | ||
| C�� | �Ҷ����ͱ����� | D�� | 1��1һ���������1��2һ�������� |
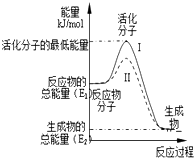 ��1����֪��
��1����֪��