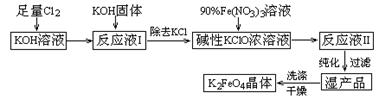
��Ŀ����
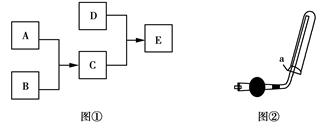
����������һ���ķ����,��ijЩ������ˮ��Ӧ������з����ͼʾ��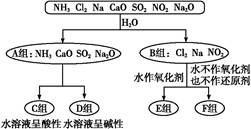
���������ѧ��֪ʶ,��Ҫ�����:
��1��������һ����������ֳ�A��B������ݣ����� ��
��2��F�������ʳ���Cl2��������������ѧʽ����
��3��A���е�CaO��������ʳƷ��װ���еĸ����,CaO��������������Ϊ�����������������գ���
�ٽ���������ڼ���������ۼ�ܼ��Ը����
CaO����������������� ���û�ѧ����ʽ��ʾ����
��4��D����NH3��ˮ��Һ��������,�õ��뷽��ʽ��ʾ��������Ե�ԭ��:�� ��
��5����Al3+�Ʊ�Al��OH��3,��ò�ѡ��D���е�NaOH��Һ,˵������:�� ��
��1���Ƿ���������ԭ��Ӧ����2��NO2
��3���٢ڢܡ�CaO+H2O Ca��OH��2
Ca��OH��2
��4��NH3��H2O N
N +OH-
+OH-
��5������NaOH��Һ���ܽ����ɵ�Al��OH��3����
����

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д��п�Ժ��ѧ�����Ƶľ�����ϡ��������������������ں˴Ź�����Ӱ��ҽҩ���й㷺��;���������̵IJ�����������ͼ��ʾ��
FeCl3��6H2O  FeOOH
FeOOH  ��������������
��������������
�������������������
| A�����������������ɷ�ɢ��ˮ�У�����FeCCl3��Һ�ķ�ɢ��ֱ���൱ |
| B�������������������д��ԣ�����Ϊҩ�������������Ƽ��� |
| C���ڷ�Ӧ���л����������ÿ����Ǵٽ��Ȼ���ˮ�� |
| D����Ӧ�ڵĻ�ѧ����ʽ��6FeOOH+CO=2Fe3O4+3H2OʮCO2 |
���������г��õ�����������������ᡱָ���ᡢ��������ᣬ�����ָ�ռ�ʹ��
��1�������ʵķ���Ƕȿ�����ǡ����һ�������� �������ʵĻ�ѧʽ����
��2�������ᡱ�롰���֮��ķ�Ӧ�����û�ѧ����ʽ��ʾ�������������ʱ�����������ӷ���ʽ��ʾȴֻ����������д�����������ӷ���ʽ���������ʱ�� �� ��
��3�������ᡱ�������ܽ�����ͽ�����������п�״�����ڳ���ʱ��ȫ����������Ũ������� ��
| A��Au | B��Cu | C��Al | D��Fe |
�� ����Na2CO3���� ����NaHCO3��
��������ʮ�����ʣ���H2 ���� ��CaO ��CO2 ��H2SO4 ��Ba(OH)2 �ߺ��ɫ����������Һ�� �ఱˮ ��ϡ���� ��Al2(SO4)3
��1�����������ʰ����ʵķ������д����Ŀհ״��������ʱ�ţ���
| ����� | �������� | ������ | ��Һ | ���� | ����� |
| ���ڸ�������� | | | | | |
��2������ʮ������������������֮��ɷ������ӷ�Ӧ��H����OH����H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________��
��3������ˮ�еĵ��뷽��ʽΪ_______________________________________________��
��4�������Ģ�ͨ�����Һ�з�Ӧ�����ӷ���ʽΪ_______________________________��
��5������ᷢ����Ӧ�Ļ�ѧ����ʽΪ��Al + 4HNO3��Al(NO3)3 + NO�� + 2H2O���÷�Ӧ����������____________���ѧʽ�����������뻹ԭ�������ʵ���֮����___________������5.4g Al������Ӧʱ��ת�Ƶ��ӵ����ʵ���Ϊ____________���÷�Ӧ�����ӷ���ʽΪ_____________________________
�����й���������ȷ����
| A��������ˮ�ĵ����һ����������� |
| B��ǿ�������ˮ��Һ�еĵ�������Dz������ |
| C��������ˮ�ĵ����һ����ǿ����� |
| D��ǿ����ʵ�ˮ��Һ��������һ�����������ˮ��Һ�ĵ�������ǿ |