��Ŀ����
��ͼΪһ��������ͼ����С����ͼ���������ҷֱ�д��H2��CO2��Na2O��NaCl��FeCl3�������ʣ�ͼ���������������ʾ��ɹ���Ϊһ�࣬�ཻ����������A��B��C��DΪ����Ӧ�ķ������ݴ��š�
��ش��������⣺
(1)�뽫�������ݴ���������Ӧ��������
( )�������ʶ����ǵ����
( )�������ʶ����ƵĻ�����
( )�������ʶ���������
( )�������ʶ�����
(2)�ýྻ���ձ�ȡ��������ˮ���þƾ��Ƽ��������ڣ����ձ�����μ���
1 mol��L-1��ͼ��һ�ֻ������ˮ��Һ�����Ƶ�һ�ֺ��ɫ���塣
�ٷ�Ӧ�Ļ�ѧ����ʽΪ��________________________________________________��
������ýϼķ����жϽ����Ƿ��Ʊ��ɹ���
___________________________________________________________
����ý�������μ������ᣬ�����һϵ�б仯��
a���ȳ��ֺ��ɫ������ԭ����___________________________________________
b�������ɫ�����ܽ⣬�˷�Ӧ�����ӷ���ʽ��____________________________________
(1)A C B D
(2)��FeCl3��3H2O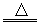 Fe(OH)3(����)��3HCl
Fe(OH)3(����)��3HCl
�����÷�ɢϵ�ܲ��������ЧӦ�����Ʊ��ɹ��������ɹ�
��a.�����HClʹFe(OH)3���巢���۳�����Fe(OH)3����
b��Fe(OH)3��3H�� =Fe3����3H2O
����

���мӵ��ֵĺ��岻��ָ��Ԫ�ء�����
| A���������۱��� | B���Ƶ���ɫ��ӦΪ��ɫ |
| C����Ȫˮ�к��иơ�þ�ȿ����� | D����ͬ���������к��ס����� |
�������̽��Ҫ�õ����ѧ֪ʶ��
(1)�±��г������������Ħ���������ڱ�����д����Ħ�����������������
| ���� | �������ͯ���� | �������������� | �л������� |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ�������������(ָ�ᡢ��Ρ������������������) | | | |
(2)��������Ʋ⣬����Ħ�������ܽ�����________________________
(����ܡ������ܡ�)��
(3)�����е�Ħ����̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ��ʵ�����Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��
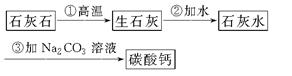
��д�������������йط�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��________________________________________________��
��________________________________________________��
��________________________________________________��
(4)��������ʯ��ʯ��ԭ��(�����Լ���ѡ)�����ʵ�����Ʊ�̼��Ƶ���һ��ʵ�鷽��������(3)��ʾ�������ʵ�鷽��������ͼ��ʾ������
ʯ��ʯ�D��
����Ƶķ������ŵ�Ϊ��________________________________��
(5)�����������Ƿ���̼��Ƶ�ʵ�鷽���ǣ�__________________��
�����£����и��������ܴ����������
| A��ϡ�����У�K����Mg2+��AlO2����S2O32�� |
| B��Na2S��Һ�У�SO42-��K����Cl����Cu2�� |
C��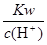 =10-13mol��L-1��Һ�У�Fe3+��NH4����Mg2+�� SO42- =10-13mol��L-1��Һ�У�Fe3+��NH4����Mg2+�� SO42- |
| D��ͨ�����CO2����Һ�У�Na+��ClO����CH3COO����HCO3�� |

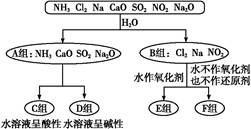

 ���۰��ף���������
���۰��ף��������� ����
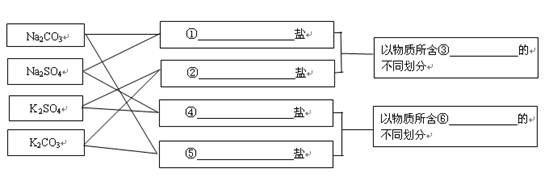
���� Cl����
Cl���� Cl�������
Cl�������